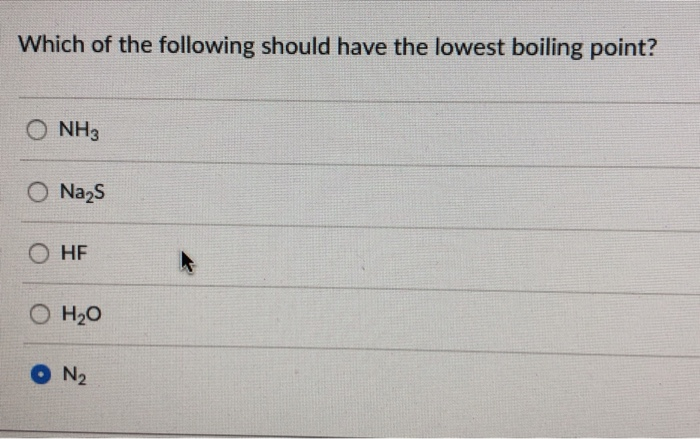
Which Of The Following Should Have The Lowest Boiling Point

Hey there, fellow life enthusiasts! Ever found yourself staring into the bubbling abyss of a science textbook, or maybe just pondering life's mysteries while waiting for your kettle to boil? Today, we’re diving into something a little bit… airy. We’re going to chat about boiling points. Not in a dry, lecture-hall kind of way, but with a gentle breeze of curiosity and a sprinkle of everyday magic. Think of it as a cozy chat over a cup of tea, but with a dash of molecular intrigue.
So, the big question, the one that might just keep you up at night (or at least pique your interest during a leisurely scroll): Which of the following should have the lowest boiling point? Now, I know what you’re thinking. "Boiling points? Isn't that for chemists and rocket scientists?" And yes, they definitely get their fair share of boiling point action. But guess what? You're interacting with boiling points all the time. From the morning coffee ritual to that perfectly steamed broccoli, it's woven into the fabric of our daily lives. So, let's demystify this a bit, shall we?
First off, what exactly is a boiling point? In simple terms, it's the temperature at which a liquid turns into a gas (or vapor) when it’s heated up. It’s like the liquid's ultimate "escape velocity" into the atmosphere. This happens when the liquid's internal pressure is equal to the surrounding atmospheric pressure. When those two forces balance, the molecules in the liquid gain enough energy to break free from their neighbors and float around as a gas. Pretty neat, right?
Now, why do some substances boil at way lower temperatures than others? It all boils down to intermolecular forces. Don't let the fancy name scare you! These are just the little "sticky" forces that hold molecules together. Think of them like tiny magnets or Velcro patches between the molecules. The weaker these forces are, the less energy (heat) it takes to break them apart and make the substance boil. The stronger the forces, the more heat you need. It's like trying to pull apart a bunch of friends holding hands – if they're just lightly touching, it's easy. If they're in a super-tight hug, it takes a lot more effort!
So, let's get to the fun part: the lineup! Imagine you’re presented with a few common substances, and you have to make an educated guess about which one will be the first to say "see ya!" and become a gas. Let's conjure up a hypothetical scenario, a little quiz for your brain. Picture these in your mind, maybe while you're out for a walk, enjoying the fresh air:
Our Imaginary Contestants:
- Water (H₂O): The star of our kitchens and our showers.
- Ethanol (C₂H₅OH): The spirit in your evening drink.
- Methane (CH₄): The main ingredient in natural gas.
- Ammonia (NH₃): A pungent chemical, often used in cleaning products (though you probably don't want to boil that at home!).
Okay, drumroll, please! Which one of these do you think has the lowest boiling point? If you’re guessing Methane, you’re on the right track! Let's break down why.
When we talk about boiling points, we’re really talking about the strength of attraction between individual molecules of a substance. Different molecules have different "personalities," if you will, and these personalities dictate how strongly they cling to each other.
Let's start with our familiar friend, water (H₂O). Water molecules are like tiny lovebirds. They form something called hydrogen bonds. These are quite strong intermolecular forces. Imagine each water molecule having little hands that reach out and grab onto other water molecules with extra enthusiasm. This is why water has a relatively high boiling point for its size – 100°C (212°F). It takes a good amount of energy to pry those lovebirds apart!
Next up, ethanol (C₂H₅OH). Ethanol is the alcohol found in beer, wine, and spirits. It’s also similar to water in that it can form hydrogen bonds, but it has a larger, more "hydrophobic" (water-hating) part. While it does have hydrogen bonding, it's a bit weaker than in pure water because of the ethyl group (the C₂H₅ part). Think of it as a slightly less enthusiastic hug compared to water. As a result, ethanol’s boiling point is lower than water’s, around 78.37°C (173.07°F). So, it's easier to get ethanol molecules to dance around freely.
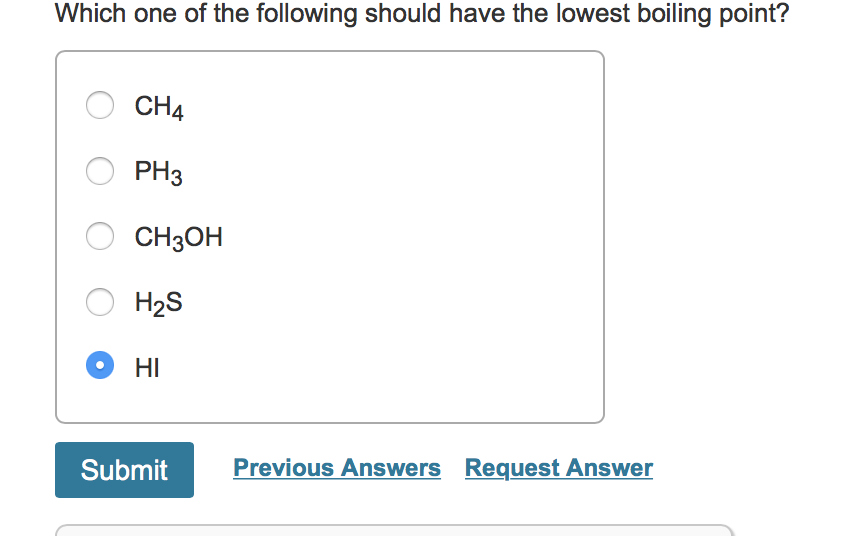
Now, let’s venture into the realm of the truly chilly. Methane (CH₄) is the simplest hydrocarbon. It’s a small molecule, and the forces between methane molecules are very weak. They’re primarily what we call van der Waals forces, specifically London dispersion forces. These are the weakest type of intermolecular force. Imagine methane molecules as tiny marbles that just occasionally bump into each other. There's very little "stickiness" holding them together. Because these forces are so weak, it takes very little energy to overcome them. Methane boils at a frosty -161.5°C (-258.7°F). Yep, that’s a really cold temperature!
Finally, ammonia (NH₃). Ammonia is interesting because it can also form hydrogen bonds, similar to water. However, the hydrogen bonding in ammonia is not as strong as in water, but stronger than in methane. Its boiling point is around -33.34°C (-28°F). So, while it’s much colder than water and ethanol, it’s significantly warmer than methane.
So, when we compare our hypothetical lineup: Water (100°C), Ethanol (78.4°C), Ammonia (-33.3°C), and Methane (-161.5°C), it’s clear that Methane takes the prize for the lowest boiling point. It’s the ultimate free spirit, ready to become a gas at the slightest encouragement (or rather, the slightest increase in temperature from its already frigid state!).
Why Does This Matter in the Real World?
Understanding boiling points isn’t just for lab coats and science fairs. It’s subtly influencing our world in countless ways. Think about:
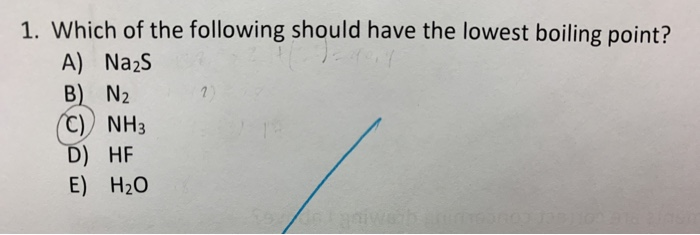
- Cooking: We boil water at 100°C for pasta, but we don't want our olive oil (which has a much higher boiling point) to vaporize when we’re sautéing. The difference in boiling points allows us to cook effectively.
- Refrigeration: Refrigerants are chemicals designed to have very low boiling points. They absorb heat from inside the fridge as they boil, thus cooling the interior. Then, they are compressed and cooled to become liquid again, ready for another cycle. It's a continuous boiling and condensing dance!
- Fuel Efficiency: Different fuels have different vaporization points. This affects how they burn and how efficiently they can be used in engines. Natural gas, primarily methane, needs to be kept very cold to remain liquid for transport, highlighting its low boiling point.
- Everyday Products: Aerosol cans, like hairspray or deodorant, rely on propellants that have low boiling points. When you press the nozzle, the pressure is released, and the propellant boils and expands, pushing the product out.
It’s also fascinating to think about the cultural side. For centuries, humans have used boiling as a purification method, whether it’s boiling water to make it safe to drink or boiling down sap to make maple syrup. Each substance has its own temperature threshold, its own moment of transformation. This is elegantly reflected in how different cultures have developed different culinary traditions based on the boiling points of local ingredients.
Have you ever seen liquid nitrogen in action? It’s pure fun and a spectacular demonstration of extremely low boiling points! Liquid nitrogen boils at -196°C (-320°F). It’s so cold that it instantly freezes anything it touches, creating dramatic clouds of vapor as it boils off into the air. It’s a constant reminder of how much variation there is in the molecular world around us, right beneath our everyday experiences.
Consider the humble balloon. The air inside is mostly nitrogen and oxygen. These gases have very low boiling points, far below freezing. That's why they remain gases even on a cold winter day. If you were to try and "boil" the air in a balloon (which, please don't!), you'd need incredibly extreme temperatures. It’s a subtle but constant presence of states of matter dictated by these fundamental properties.

Think about how we describe things. We might say something is "hot-headed" or "cool as a cucumber." These are metaphors, of course, but they stem from our intuitive understanding of temperature and energy. The boiling point is a scientific interpretation of that intuitive understanding – a precise measurement of how much energy a substance needs to transition from a liquid hug to a gaseous dance.
Even something as simple as a hot air balloon relies on the principle that heating air causes it to expand and become less dense, not directly boiling, but a related concept of molecular energy. The principle is that energy changes the behavior of molecules, and boiling is one of the most dramatic demonstrations of that change.
So, the next time you’re sipping on your favorite beverage or watching steam rise from a pot, take a moment to appreciate the silent, unseen world of molecular interactions that makes it all possible. The journey from a clinging liquid to a free-spirited gas is governed by forces that are both fundamental and fascinating. And remember, that chilly methane, with its ability to become a gas at the drop of a thermometer, is a prime example of how vastly different these molecular personalities can be.
A Little Reflection
It’s funny, isn't it? We spend so much of our lives navigating the world of observable phenomena – the taste of our food, the warmth of the sun, the colors we see. But underneath all of that, there’s this incredible, invisible dance of molecules, each with its own unique energy requirements and attractions. Understanding something like a boiling point, even in a simple, lighthearted way, gives us a tiny peek behind the curtain. It reminds us that even the most commonplace things are governed by profound and elegant principles. It’s a little bit like realizing that the effortless grace of a ballet dancer is the result of years of intense training and precise muscle control. We get to enjoy the beauty of the finished performance, while the underlying science, or in the dancer’s case, the rigorous practice, happens mostly out of sight. And that, in itself, is pretty cool.
