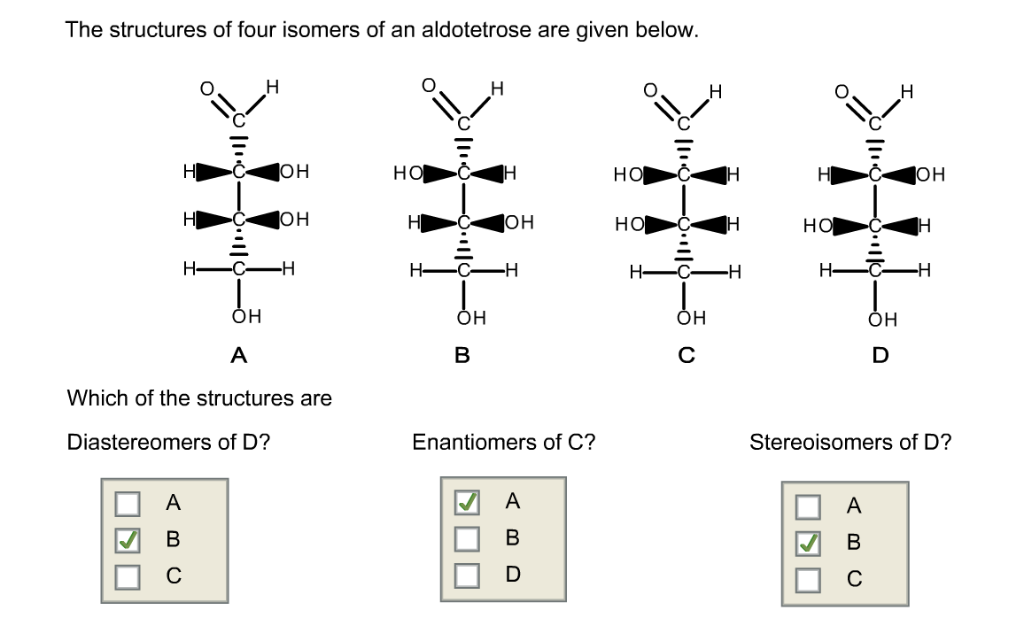
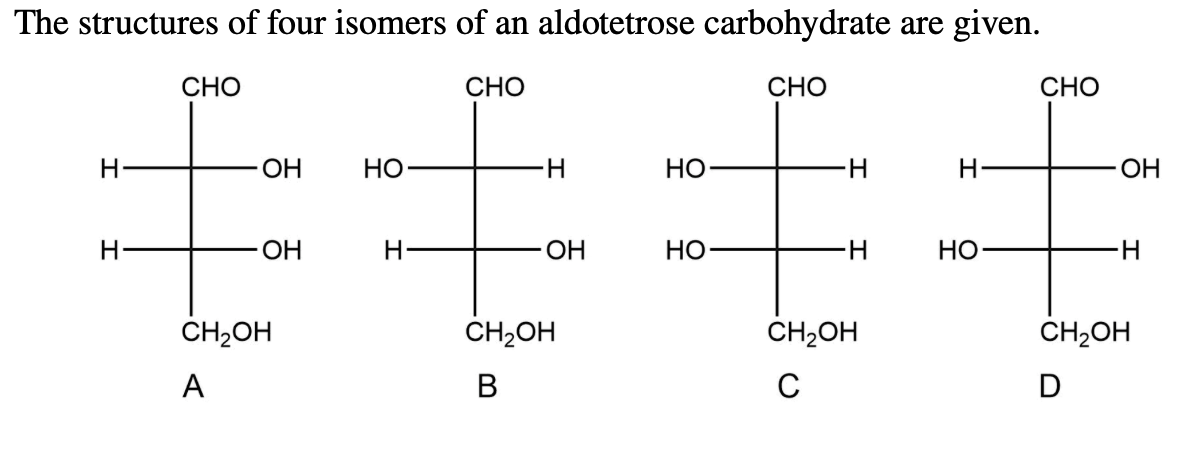
The Structures Of Four Isomers Of An Aldotetrose Are Given.
Hey there, science explorers! Ever feel like you're looking at something, and then you look again, and it's almost the same, but somehow… different? Like when you see two twins who look super alike but have totally different personalities? Well, guess what? Molecules can pull that same kind of trick on us! Today, we're diving into the fascinating world of sugars, specifically a group called aldotetroses. Don't let the fancy name scare you – it just means a four-carbon sugar with a specific kind of chemical group at one end. Pretty straightforward, right? But here's where it gets wild: even with just four carbons, we can arrange them in different ways, creating molecules that are the same formula-wise but different in their 3D shape. Think of it like having the same set of Lego bricks, but building a race car one time and a spaceship the next. Cool, huh?
So, what's an aldotetrose anyway? Imagine a tiny, tiny chain of four carbon atoms. On one end, there's a special group called an aldehyde group (think of it as a little functional hook). And the rest of the carbons are hanging out with hydrogen atoms and some hydroxyl groups (which are like little sticky OH bits). The basic recipe is the same for all of them: C4H8O4. But the magic, the real interesting part, is how those little atoms are arranged in space. It's all about the stereochemistry, which is just a fancy way of saying the 3D arrangement of atoms in a molecule.
These four-carbon sugars, or aldotetroses, have a couple of "chiral centers." Now, a chiral center is like a carbon atom that has four different things attached to it. Imagine a dancer spinning around with their arms and legs in four unique positions. That carbon atom is a chiral center. And if a molecule has one or more chiral centers, it can exist as different stereoisomers. These are like mirror images that you can't superimpose, kind of like your left and right hands. They look similar, but they’re not exactly the same. It's mind-bending stuff!
For aldotetroses, with two chiral centers, we can have up to 2^2 = 4 different stereoisomers. Yep, just four! Isn't that neat? It’s like nature’s way of giving us a small, manageable set to play with. And the structures of these four isomers are given to us. Let's break down what that might look like. Imagine you have a sheet of paper, and you're drawing these sugar molecules. We often represent them using something called a Fischer projection. It’s a way to flatten out a 3D molecule onto a 2D surface, showing the horizontal lines as coming out at you and the vertical lines going back into the page.
So, all four of these aldotetroses will have that aldehyde group at the top. Then, you'll have the two chiral carbons in the middle, and finally, the last carbon at the bottom. The difference between them lies in the arrangement of the –OH (hydroxyl) groups on those two middle carbons. It's like having two knobs you can turn, and each combination gives you a unique molecule.
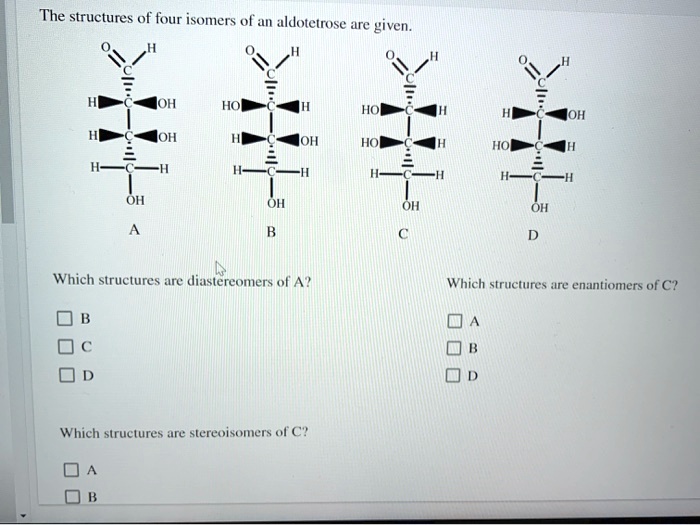
Let's give them some names, shall we? Because, you know, scientists love to name things! The four main aldotetroses are D-erythrose, L-erythrose, D-threose, and L-threose. Pretty cool names, right? They hint at their relationships. The 'D' and 'L' refer to their relationship to a master sugar called D-glyceraldehyde, but don't worry too much about that for now. The key difference is between 'erythrose' and 'threose'.
Imagine you're looking at those two middle carbons. In erythrose, the –OH groups on both chiral carbons are on the same side of the Fischer projection. It's like if you had two mirrors facing each other and saw a reflection of a reflection. They’re arranged symmetrically. So, D-erythrose and L-erythrose are mirror images of each other. If you tried to lay one perfectly on top of the other, it wouldn't work – just like your left glove won't fit perfectly on your right hand. They are enantiomers.
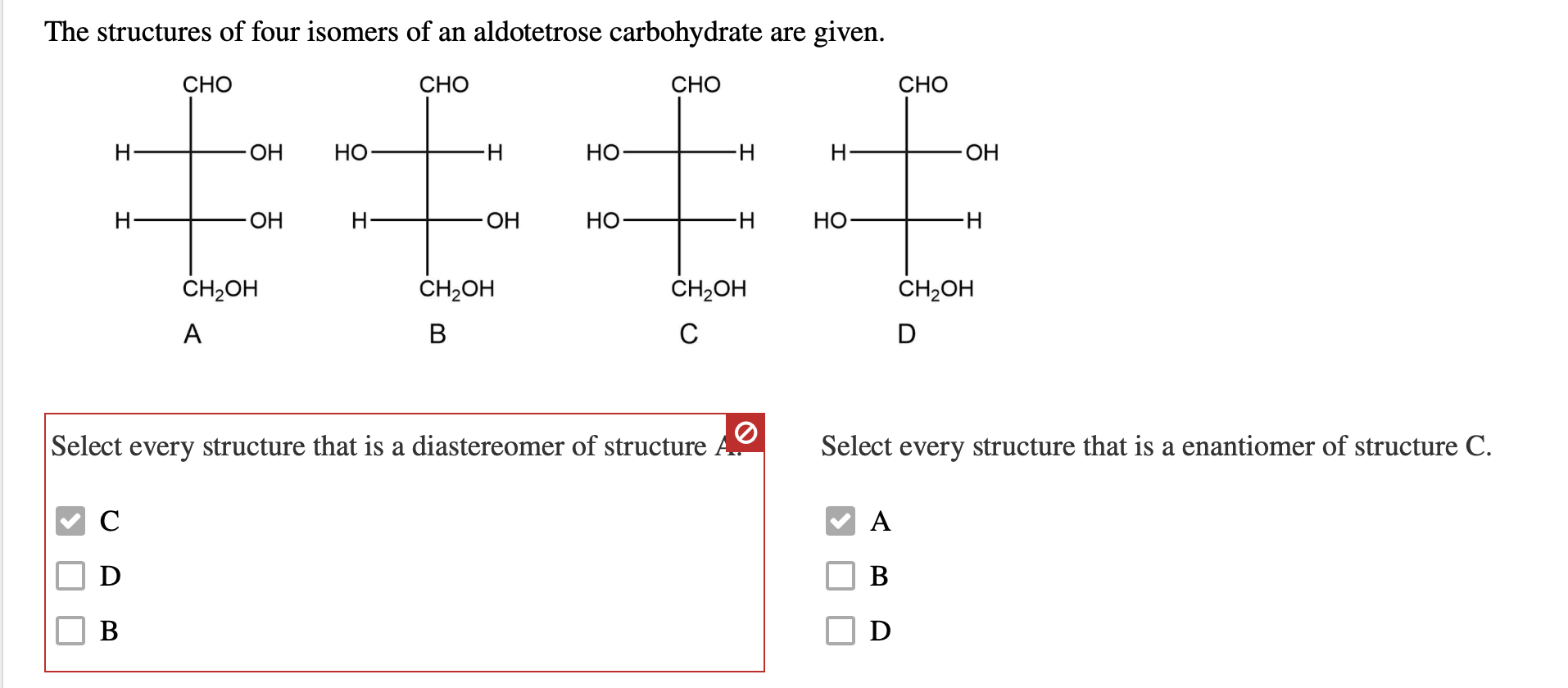
Now, for threose, the –OH groups on those two middle chiral carbons are on opposite sides. It’s like having one knob turned left and the other turned right. This arrangement is different from erythrose. And just like with erythrose, D-threose and L-threose are also mirror images of each other, making them enantiomers. So, D-erythrose and L-erythrose are a pair of enantiomers, and D-threose and L-threose are another pair of enantiomers.
But here's the really mind-bending part: erythrose and threose are not mirror images of each other. They are called diastereomers. Think of it like this: D-erythrose and D-threose are like cousins. They share some structural similarities because they are both aldotetroses and have the same number of carbons and functional groups, but their 3D arrangements are different enough that they aren't perfect mirror images. They’re like siblings from different parents, if that makes sense! They have different properties and behave differently in chemical reactions.
Why is this all so cool? Well, these tiny differences in shape can have huge impacts in biology. Our bodies are incredibly sensitive to the 3D shapes of molecules. Enzymes, for example, are like highly specific locks, and they can only fit the right key (the right sugar shape). So, even though D-erythrose and D-threose have the same chemical formula, one might be perfectly used by an enzyme in your body, while the other might be completely ignored or even cause problems. It’s like trying to fit a square peg into a round hole – it just doesn’t work.
This concept of stereoisomerism is fundamental to understanding how life works at the molecular level. It explains why certain drugs work better than others, why some natural compounds have potent effects, and how our cells recognize and process different molecules. It’s like a secret code that dictates how molecules interact. The fact that just changing the spatial arrangement of a few atoms can lead to such different outcomes is truly a testament to the elegance and complexity of chemistry and biology.
So, next time you're enjoying a sweet treat, remember that the sugar molecules making it sweet are not just simple chains. They have intricate 3D structures, and those structures matter. The world of isomers is a constant reminder that even in the smallest building blocks of life, there’s a universe of variation and specificity waiting to be discovered. It’s a reminder that sometimes, the devil (or the magic!) is truly in the details, or rather, in the spatial arrangement of those details!
