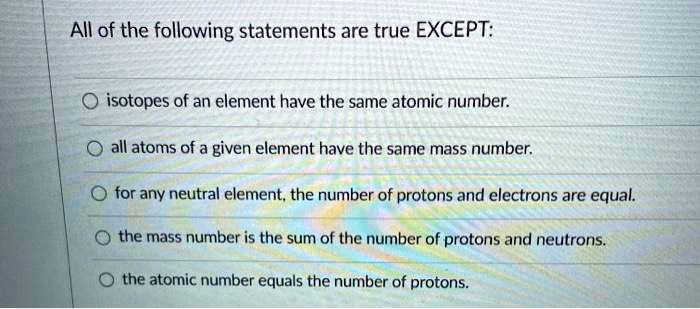
All Of The Following Are True Statements About Atoms Except
Alright folks, gather 'round, grab a virtual croissant, and let's dive into the wonderfully weird world of atoms. Now, you might be thinking, "Atoms? That sounds like something my high school science teacher droned on about while I was secretly drawing superheroes in my notebook." But stick with me, because these tiny little things are the absolute building blocks of everything. Seriously. Your coffee, your phone, that weird dust bunny under your sofa – all atoms! And today, we're playing a game, a bit like a science-themed "Which one doesn't belong?" The question is: All of the following are true statements about atoms except... Let's have some fun with this, shall we?
So, let's start with some absolute, undeniable truths about our atomic buddies. First off, atoms are ridiculously, astronomically small. Like, if a golf ball were the size of the Earth, an atom would be the size of a tiny, microscopic speck of dust. Yeah, try visualizing that. My brain pretty much just blue-screened trying to comprehend it. It's like trying to count all the grains of sand on all the beaches in the world, and then dividing that by a gazillion. Mind-boggling, right?
Now, inside these itty-bitty wonders, we have even smaller things. We're talking about protons, neutrons, and electrons. Think of it like a cosmic disco ball. The protons and neutrons are chilling in the center, the nucleus – the dance floor, if you will. They're pretty hefty, these two. Then you've got the electrons, zipping around the outside like hyperactive toddlers at a birthday party. They're way lighter, and they're constantly on the move. It's a whole lot of hustle and bustle in a space that’s practically invisible.
Here’s a fun fact for you: most of an atom is actually empty space! Seriously. If you could somehow strip away all the empty space from all the atoms in all the people on Earth, the entire human race could fit into the volume of a sugar cube. A single, solitary sugar cube. So next time you're feeling a bit crowded, just remember, scientifically speaking, we're all just a bunch of empty space pretending to be solid. Awkward!
And get this: atoms are constantly in motion. They're not just sitting there like little statues. They're vibrating, jiggling, bouncing around like they've had too much caffeine. This constant movement is what gives things their temperature. The hotter something is, the more its atoms are doing the Macarena. The colder it is, well, they’re probably just swaying gently, contemplating their existence.

The Case of the Misbehaving Atom
Now, because this is a "except" game, we're going to encounter some statements that sound almost true, but have a little twist of deception. It's like your friend telling you they totally finished that assignment, but then you see they haven't even opened the document. Suspicious, right?
Let’s imagine a statement like: "Atoms are so stable, they never change or decay." Sounds plausible, right? Atoms are pretty darn persistent. But here's the catch: radioactive atoms exist! These are the rebels of the atomic world. They're a bit unstable, and they can spontaneously transform into other elements, releasing a burst of energy in the process. Think of it as an atom having an existential crisis and deciding to become something else entirely. It's a bit dramatic, but hey, it happens!

Another one to watch out for: "All atoms of a specific element are exactly identical." This is where things get a little fuzzy, like a poorly focused photograph. While most atoms of, say, oxygen are pretty much the same, there are things called isotopes. Isotopes are like the slightly different cousins of an element. They have the same number of protons (which is what defines the element, like its official ID), but a different number of neutrons. So, they're still oxygen, but they’re a little bit heavier or lighter. It’s like having twins who are both doctors, but one specializes in cardiology and the other in neurology. Same profession, slightly different focus.
And then there's the idea that atoms are just tiny, solid balls. Like miniature marbles. This is an old-school way of thinking about them, a bit like believing the Earth is flat. While the nucleus is pretty dense, remember all that empty space we talked about? It's more like a fuzzy cloud of probability where the electrons might be. It’s less like a solid marble and more like a very energetic, very confusing swarm of gnats, but you can’t really see the gnats, you just know they’re somewhere around. Bewildering!

The Electron Escapade
Let’s talk about electrons for a second, because they’re the real show-offs. Electrons have a negative charge. Protons have a positive charge. And neutrons? They’re neutral, like a diplomat at a tense negotiation. Opposite charges attract, remember? That’s why the positively charged nucleus and the negatively charged electrons like to hang out together, even though the electrons are constantly trying to escape. It's a cosmic dance of attraction and repulsion.
Now, here's a real stumper: "Electrons orbit the nucleus in perfect, predictable paths, like planets around a sun." If only it were that neat and tidy! Electrons don't orbit in nice, neat circles. They exist in what scientists call orbitals, which are more like regions of space where there's a high probability of finding an electron. It’s more like a foggy cloud than a precise racetrack. They’re not “here” or “there” in the way we usually think of things. They’re more like a Schrödinger's Cat situation, existing in multiple possibilities at once until we try to pin them down.
So, to recap our game of "Except": We know atoms are tiny, made of protons, neutrons, and electrons, mostly empty space, and always moving. We also know that some atoms can change (radioactivity), and not all atoms of an element are exactly identical (isotopes). And those electrons? They’re not exactly following a cosmic GPS system. They’re more… energetically distributed. The statement that’s NOT true is the one that paints a picture of absolute predictability and unchanging solidity for all atoms, everywhere, all the time. Because in the world of the infinitesimally small, things are rarely that simple. They’re wonderfully complex, a bit chaotic, and frankly, a lot more interesting than a perfect, predictable orbit. It’s like trying to find a single definition for "awesome" – you just can’t. And that, my friends, is the beauty of atoms.
