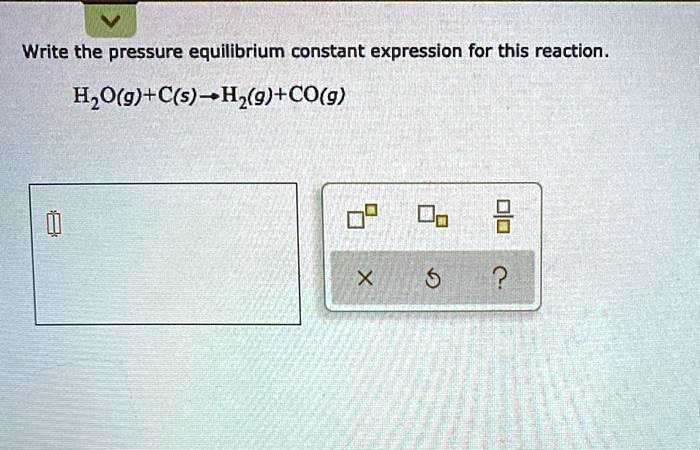
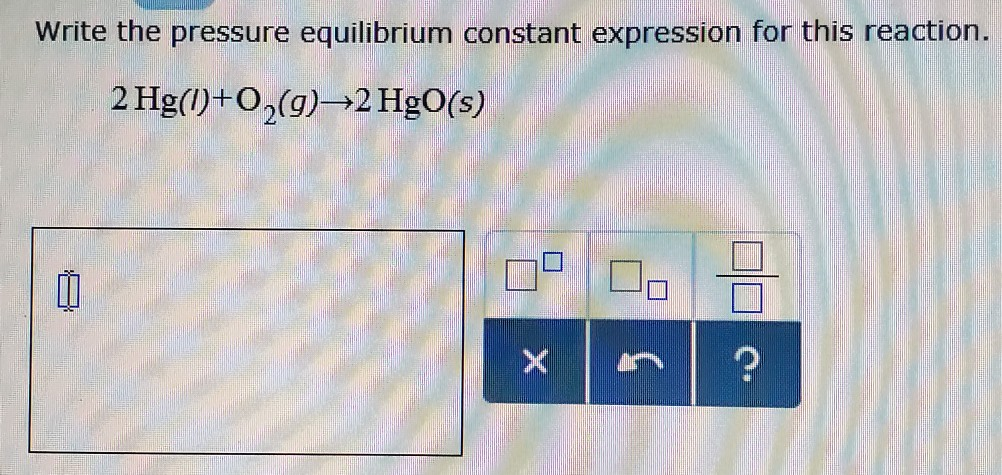
Write The Pressure Equilibrium Constant Expression For This Reaction
Alright, science adventurers, gather 'round! We're about to embark on a thrilling quest into the magical world of chemical reactions. Don't worry, no lab coats or complicated equations are required today. We're going to make this as easy and fun as baking your favorite cookies!
Imagine a bustling kitchen where a super-secret recipe is being made. Our recipe involves mixing a few ingredients to create something utterly delicious. But here's the kicker: sometimes, the delicious thing can magically turn back into the original ingredients! It's like a culinary chameleon, constantly changing its mind.
In the world of chemistry, we call this a reversible reaction. It's not a one-way street; it's a two-way avenue where things can go forward and backward. It’s a constant dance between creating and un-creating, a delightful balancing act that keeps chemists on their toes.
Now, wouldn't it be super neat if we could somehow measure how much of the delicious new stuff we have compared to the original ingredients at any given moment? Especially when the reaction has settled down, like a perfectly baked cake that's cooled and is ready to be admired. This is where our superhero, the Pressure Equilibrium Constant Expression, swoops in to save the day!
Think of it like this: imagine a party. You've invited guests (your ingredients) and they're all mingling and having a blast. Some of them are chatting and becoming new friends (forming products), while others are perhaps remembering their old pals and splitting off to talk to them again (going back to reactants). The party eventually reaches a point where the number of new friendships forming is perfectly matched by the number of old friendships re-forming. It’s a state of equilibrium, a harmonious buzz!
Our Pressure Equilibrium Constant Expression is like a secret handshake that tells us the ratio of those new friends (products) to the old pals (reactants) when the party is in full swing and has reached its peak chill. It’s a snapshot of who’s where, and in what abundance, when everything has settled into its happy equilibrium. It’s the ultimate party gossip, telling us all about the dynamics of our reaction.

Let's Get Practical (but still fun!)
So, how do we actually write this magic expression? It's not as scary as it sounds. It’s like writing down the recipe for success, but instead of listing ingredients, we’re listing their “pressures.”
First things first, we need a reaction to play with. Let’s invent a super-duper exciting one! Imagine we have a bunch of tiny, bouncy A molecules and some equally bouncy B molecules. They get together and, poof, they form some wonderful C molecules and maybe even some extra-special D molecules.
But remember our reversible nature? Those C and D molecules can also decide to break up and go back to being A and B. It’s a never-ending cycle of connection and disconnection, a testament to the dynamic nature of chemistry.

So, our epic reversible reaction looks something like this in the mystical language of chemistry:
aA + bB ⇌ cC + dD
See those little letters, a, b, c, and d? They’re like the number of people invited from each group to the party. They tell us how many of each molecule are involved in the formation or breaking apart. If there’s no little number, it just means there’s one!
Now, for our Pressure Equilibrium Constant Expression, we want to know the ratio of the "stuff we made" (products) to the "stuff we started with" (reactants). But we’re talking about pressure here, so we're focusing on the partial pressures of each gas involved in our reaction.
The Grand Formula Revealed!
Here it is, in all its glory, the magical expression! Drumroll, please…

Kp = (PCc * PDd) / (PAa * PBb)
Whoa, right? But let’s break it down. It’s less like a scary monster and more like a well-organized filing system.
On the top, we have the products – the wonderful things we've created. We have the pressure of C (written as PC) and the pressure of D (written as PD). And see those little superscripts, c and d? They’re the same numbers from our reaction equation! They mean we multiply the pressure of each product by itself that many times. So, if we had 2 C molecules, we'd have PC * PC, which is PC2. It’s like giving extra weight to the more numerous guests!
On the bottom, we have the reactants – the original ingredients. We have the pressure of A (PA) and the pressure of B (PB). And guess what? Those little superscripts, a and b, are back again! They tell us to multiply the pressure of each reactant by itself that many times. So, if we had 3 A molecules, we’d have PA * PA * PA, or PA3. It’s all about how much each participant is contributing to the equilibrium party.
The Kp is our special symbol for the Pressure Equilibrium Constant. It’s the final score, the ultimate ratio that tells us the state of our reaction when it's all chilled out. A big Kp means we have a lot more products than reactants when equilibrium is reached – our party is a huge success with tons of new friendships! A small Kp means the reactants are still dominating the scene.
It’s like saying, for every 10 molecules of A and B that started this whole shindig, we ended up with, say, 100 molecules of C and D! Or maybe, for every 100 molecules of A and B, we only ended up with a humble 5 molecules of C and D. The Kp value quantifies this.
So, there you have it! The Pressure Equilibrium Constant Expression. It’s not some intimidating giant, but rather a very clever tool that helps us understand the delicate balance of reversible reactions using the pressures of gases. It's a way to peek into the heart of a reaction and see what its preferences are when it's just hanging out, completely at ease.
Next time you see a reversible reaction, you can give a little nod to the Kp expression. It’s the unsung hero, the silent observer that tells us all the juicy details about how the chemical world likes to party and reach its perfect, balanced state. Happy expressing, future chemists! You’ve totally got this!
