Write The Full Ground State Electron Configuration For Each Element
.PNG)
Ever wondered what makes an element, well, an element? Like, why is iron a solid at room temperature, but helium is a gas that floats away if you're not careful? A big part of that is their electron configuration. Think of it like the element's personal instruction manual, telling its electrons exactly where to hang out. And today, we're going to peek inside that manual and figure out the full ground state electron configuration for each element. Sounds a bit intimidating? Don't worry, we're going to keep it super chill, like browsing through a really interesting, albeit slightly nerdy, photo album.
So, what's the big deal about electron configurations anyway? Well, it's basically how we describe where all the electrons in an atom are buzzing around the nucleus. Imagine the nucleus as the center of a tiny solar system, and the electrons are the planets. But instead of neat, predictable orbits, electrons hang out in these things called orbitals. These orbitals are like cozy little neighborhoods, each with its own capacity for housing electrons.
There are different types of these orbital neighborhoods, too. We've got the 's' neighborhoods, which are nice and spherical, like a perfectly round beach ball. Then there are 'p' neighborhoods, which are a bit more like dumbbells, usually found in sets of three, pointing in different directions. And it goes on from there with 'd' and 'f' neighborhoods, getting a bit more complex in shape, but the basic idea is the same: they're just places electrons can be.
Why is this "ground state" thing important?
The "ground state" is the most stable configuration. It’s like an element chilling out on its couch, totally relaxed. Electrons will generally try to get to the lowest energy level possible, which is what the ground state represents. They aren't jumping around or getting excited (unless you add some energy, but that’s a story for another day!). So, when we talk about the full ground state electron configuration, we're talking about the default, lowest-energy arrangement for all the electrons in a neutral atom.
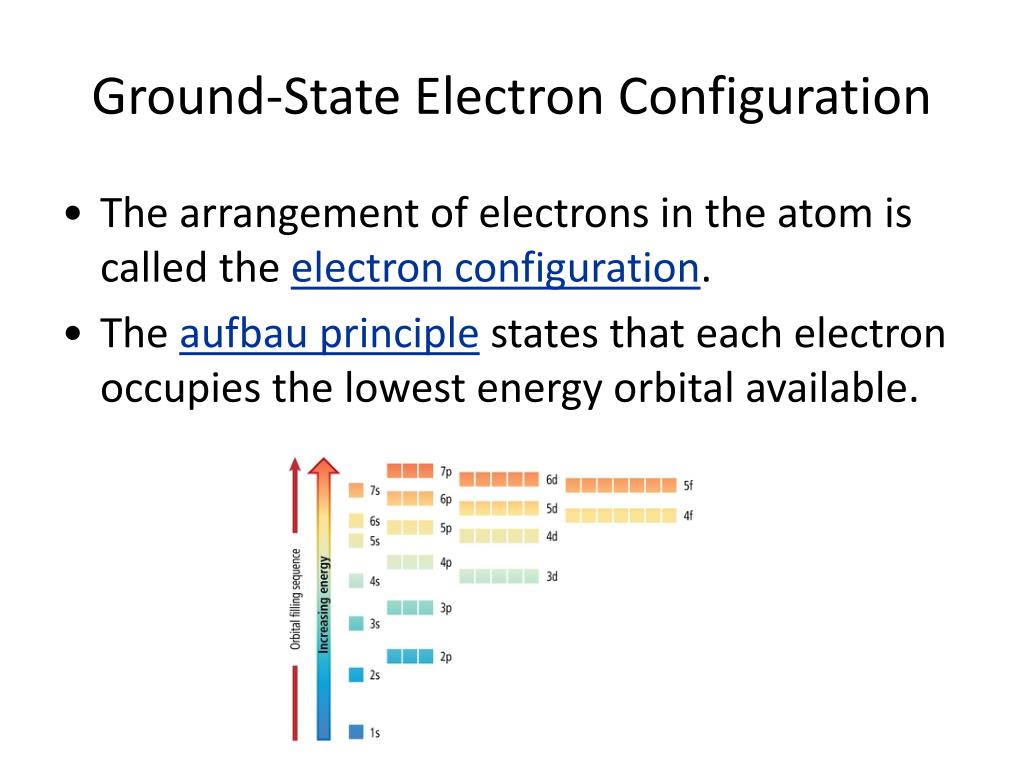
Now, how do we actually write these configurations down? It's like filling up those orbital neighborhoods. Electrons fill the lowest energy levels first, kind of like filling up the best seats at a concert before moving to the back row. We use a special shorthand notation. For example, the '1s' orbital can hold a maximum of 2 electrons. So, we write it as 1s². The '2s' orbital also holds 2, so that's 2s². The '2p' orbitals can hold up to 6 electrons, so you'll see 2p⁶. It's like saying, "Okay, the first energy level's 's' neighborhood is full with 2 electrons, and the second energy level's 's' neighborhood is also full with 2, and its 'p' neighborhoods are totally packed with 6!"
This whole system follows a few rules, but the most important one for us today is just filling them up in order of increasing energy. Think of it like a cosmic conveyor belt, moving electrons from one orbital neighborhood to the next as they get added to an element.

Let's dive into some examples!
We’re going to go element by element, from the simplest to the more complex. It’s a journey, and trust me, it gets pretty fascinating.
First up, the granddaddy of them all: Hydrogen (H). It’s got just one electron. Where does it go? The lowest energy orbital, of course! So, its configuration is simply 1s¹. Easy peasy.
Next, Helium (He). It has two electrons. The 1s orbital can hold two, so both go there. That means Helium’s configuration is 1s². The first energy level's 's' neighborhood is officially full!
Then we have Lithium (Li). It has three electrons. The 1s orbital is already packed with 2 (from Helium). So, the third electron has to move up to the next energy level, the second energy level, and it goes into the 's' orbital there. Thus, Lithium is 1s²2s¹. See? The first level is full, and we're starting to fill the second.

Beryllium (Be) has four electrons. It's 1s² (like Helium), and then it adds another electron to the 2s orbital, filling it up. So, Beryllium is 1s²2s². Both the 1s and 2s neighborhoods are now occupied.
Now for the 'p' orbitals! Boron (B) has five electrons. It’s 1s²2s² (like Beryllium), and the fifth electron has to go into the next available orbital, which is a 'p' orbital in the second energy level. So, Boron is 1s²2s²2p¹. It’s like the first two neighborhoods are full, and we've just placed one electron in the 'p' neighborhood.
We continue this pattern. Carbon (C), with six electrons, gets 1s²2s²2p². Nitrogen (N), with seven, is 1s²2s²2p³. And Oxygen (O), with eight, is 1s²2s²2p⁴. See how those 'p' orbitals are starting to get filled?
Fluorine (F), with nine electrons, is 1s²2s²2p⁵. And finally, Neon (Ne), with ten electrons, completes the second energy level: 1s²2s²2p⁶. Neon is a noble gas, and completing these outer shells is a big reason why they're so unreactive. They're perfectly content!

The adventure continues
As we move to elements with more electrons, like Sodium (Na) (11 electrons), we've filled the first two energy levels completely. The next electron has to start filling the third energy level. So, Sodium is 1s²2s²2p⁶3s¹. That last electron is in a whole new neighborhood!
This process continues, and it gets a little more complex with the 'd' and 'f' orbitals. The order of filling isn't always strictly by the number of the energy level. Sometimes, a higher-numbered energy level's 's' orbital fills before a lower-numbered energy level's 'd' orbital. It's like sometimes you grab the slightly farther seat if it's more comfortable! This is governed by what we call the Aufbau principle and Hund's rule, but the end result is a predictable filling order.
For example, Potassium (K), with 19 electrons, is 1s²2s²2p⁶3s²3p⁶4s¹. Notice how it fills the 4s orbital before the 3d orbital, even though 3 is less than 4. The 4s orbital is actually lower in energy at this point. Pretty neat, right?
And then we get to the transition metals, which fill those 'd' orbitals. Take Iron (Fe), with 26 electrons. Its configuration is 1s²2s²2p⁶3s²3p⁶4s²3d⁶. This 'd' block is where a lot of the interesting chemical properties of metals come from!
Going even further, we have the lanthanides and actinides, which fill the 'f' orbitals. These elements have really complex electron configurations, and they are responsible for some of the most exotic materials and behaviors we see in chemistry.
So, why is knowing this full ground state electron configuration for each element so cool? Because it’s the key to understanding almost everything about an element's chemical behavior. It explains why elements form bonds the way they do, what kind of reactions they participate in, and even their physical properties. It’s like having the secret code to the entire periodic table!
Think of it this way: the electron configuration is the ultimate "about me" section for each element. It tells us what its outer electrons are up to, and those outer electrons are the ones that do all the talking and interacting when elements meet. So, next time you look at an element, you can imagine its electrons, neatly arranged in their orbital neighborhoods, ready to do their thing. It's a tiny universe within each atom, and understanding its electron configuration is like getting a backstage pass to the most fundamental workings of matter.
It’s a journey of discovery, from the single electron of Hydrogen to the complex arrangements in the heaviest elements. And at its heart, it's just a way to describe how nature arranges its fundamental building blocks. Pretty awesome, if you ask me!
