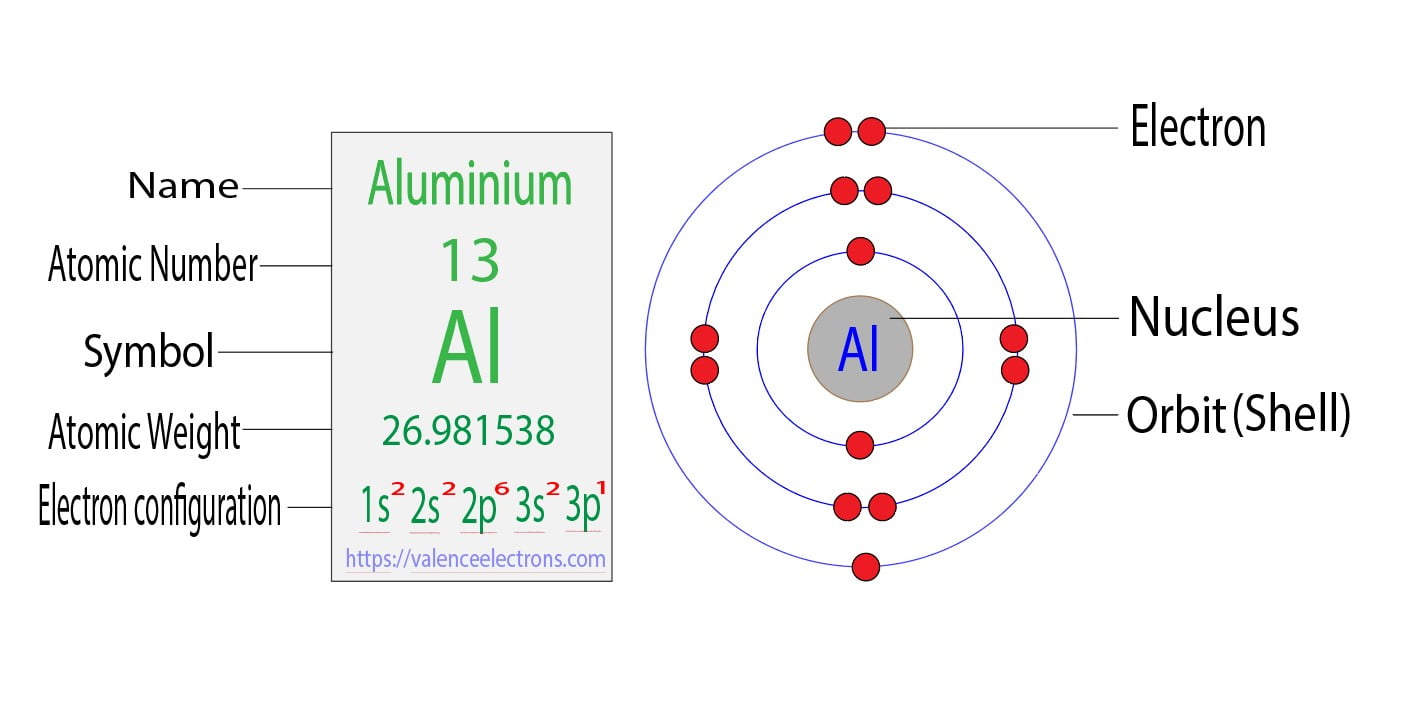
Write The Electron Configuration For A Neutral Atom Of Aluminum
Have you ever stopped to think about the tiny, invisible world that makes up everything around you? It's like a secret universe humming with activity, and today, we're going to peek into it with a friendly little explorer named Aluminum. Imagine this little guy as a building block, but instead of LEGOs, he's made of even smaller, energetic bits!
So, what's the deal with Aluminum? Well, it's a metal you probably use every single day without even realizing it! Think about the shiny foil keeping your sandwiches fresh or the lightweight cans holding your fizzy drinks. That's our pal Aluminum, working hard to make your life a little easier and a lot tastier.
Now, every atom, and that includes our Aluminum friend, has a sort of "address system" for its tiny parts. These parts are called electrons, and they love to zip around the center of the atom. Think of it like a miniature solar system, with the electrons being the planets orbiting a central sun.
This address system isn't random; it's a very organized way for the electrons to settle down. They have specific "neighborhoods" or "floors" they like to hang out in, depending on their energy level. It's like a hotel with different floors, and the electrons check into rooms based on how much energy they have.
For a neutral atom of Aluminum, we know it's got a specific number of these speedy electrons. It's not trying to gain or lose any; it's perfectly happy and balanced, just like you might be after a good night's sleep. This "neutral" state is important because it tells us exactly how many electrons we need to account for.
Now, let's get to the fun part: figuring out where all these electrons like to park themselves! It's like giving each electron its own little spot on the atomic hotel's reservation list. We start with the lowest energy "floors" first, because, well, everyone likes to be on the ground floor if they can!
The very first "floor" is called the 1s "neighborhood." It's a cozy spot and can only hold two electrons. Think of it as a tiny, exclusive club. Our Aluminum atom is so friendly, it easily fits two of its electrons right into this 1s spot.
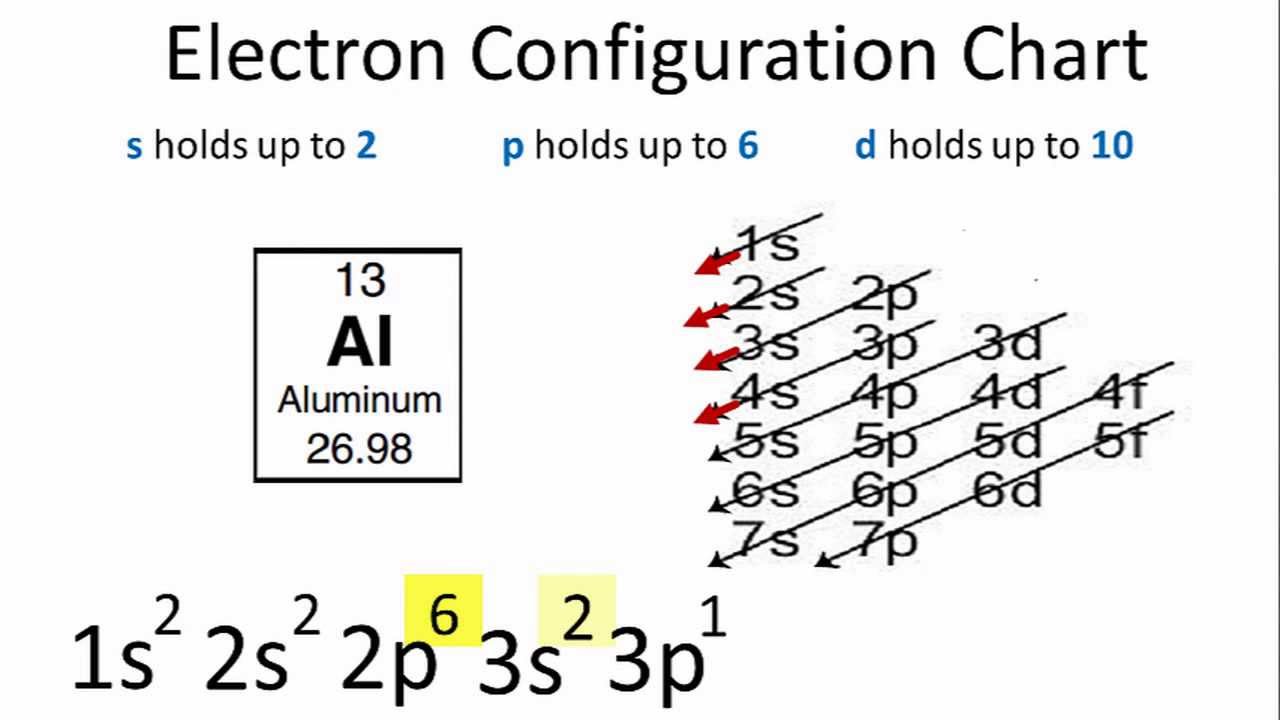
After filling up the 1s neighborhood, we move on to the next available "floor." This one is called the 2s neighborhood. It's a bit bigger and can also hold two electrons. So, two more of Aluminum's electrons happily settle in here, feeling quite content.
But wait, there's more! The second "floor" also has another type of neighborhood called 2p. This one is quite a bit larger and can accommodate up to six electrons. It's like a bustling avenue with plenty of room.
Aluminum is a pretty generous atom, so it fills up this 2p neighborhood with all six of its available electrons. Now we've accounted for quite a few of our little electron friends! They're all cozy in their designated spots, making the atom feel stable and secure.
We're not done yet! Aluminum still has some electrons to place. We move up to the next "floor," which is the third one. This floor has a neighborhood called 3s. It's similar to the 2s neighborhood and can hold two electrons.
So, two more of Aluminum's electrons find their happy place in the 3s neighborhood. They're getting a bit further from the center now, but they're still part of the happy family. It's like they've moved to a slightly nicer apartment on a higher floor.
Now, here's where things get a little interesting. We've placed quite a few electrons, but we still have a few more to go. Remember, Aluminum is a neutral atom, so it has a specific total number of electrons. We need to make sure we account for every single one of them!
The third floor also has another, even larger neighborhood called 3p. This one is like a grand ballroom and can hold up to six electrons. It's spacious and ready for more residents.
Aluminum has just enough electrons left to fill part of this 3p neighborhood. It's like a few more guests arriving at the party and finding some open spots. They settle in, making themselves at home in this spacious area.

So, if we put it all together, our Aluminum atom has its electrons organized like this: two in the 1s neighborhood, two in the 2s neighborhood, six in the 2p neighborhood, two in the 3s neighborhood, and then one in the 3p neighborhood.
But wait, that doesn't add up to the total number of electrons for Aluminum! Let's retrace our steps, shall we? It's like a fun puzzle where we make sure all the pieces fit just right. The total number of electrons for a neutral Aluminum atom is 13.
Let's try that again, shall we? We have our 1s holding 2 electrons. Then, the 2s holds another 2 electrons. The 2p is a bit bigger and can hold 6 electrons. So far, that's 2 + 2 + 6 = 10 electrons.
We still have 3 more electrons to place (13 total - 10 placed = 3 remaining). Where do they go? They move up to the next available "floor" and "neighborhood." This would be the 3p neighborhood.

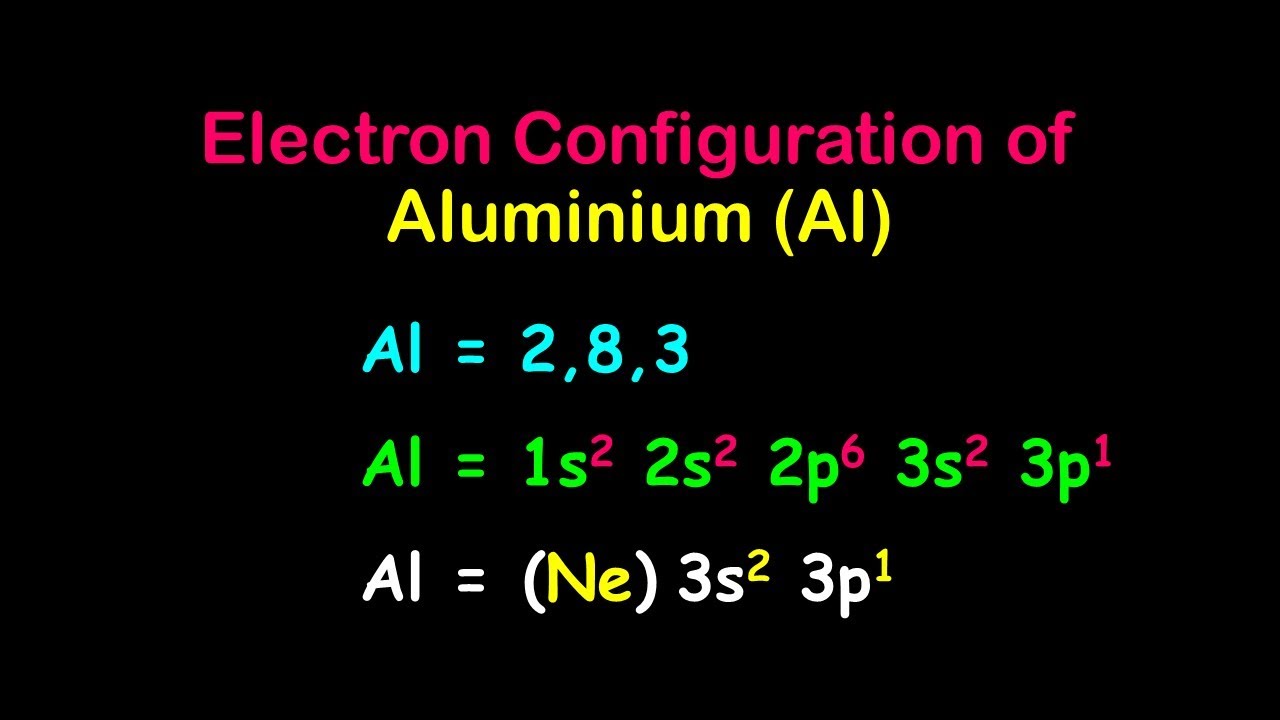
So, Aluminum's electrons will be arranged as follows:
1s² 2s² 2p⁶ 3s² 3p¹
See that little superscript number? That's just telling us how many electrons are in each of those neighborhoods. So, 1s² means there are 2 electrons in the 1s neighborhood.
Isn't it fascinating? Each electron has its designated spot, contributing to the overall personality and behavior of the Aluminum atom. It's like each electron is a little worker bee, performing its duty to keep the atom humming along.
This arrangement isn't just a silly game of musical chairs; it dictates how Aluminum interacts with other atoms. The electrons in the outermost neighborhoods are the most accessible, like the folks on the ground floor who are most likely to chat with neighbors. These outer electrons are what make Aluminum so useful in so many everyday objects.
So, the next time you unwrap a chocolate bar or reach for a cold drink, take a moment to appreciate the incredible, organized dance of electrons within that humble piece of Aluminum. It's a tiny universe of order and energy, working silently to make your world a little brighter and a lot more convenient. It's a small wonder, indeed!
