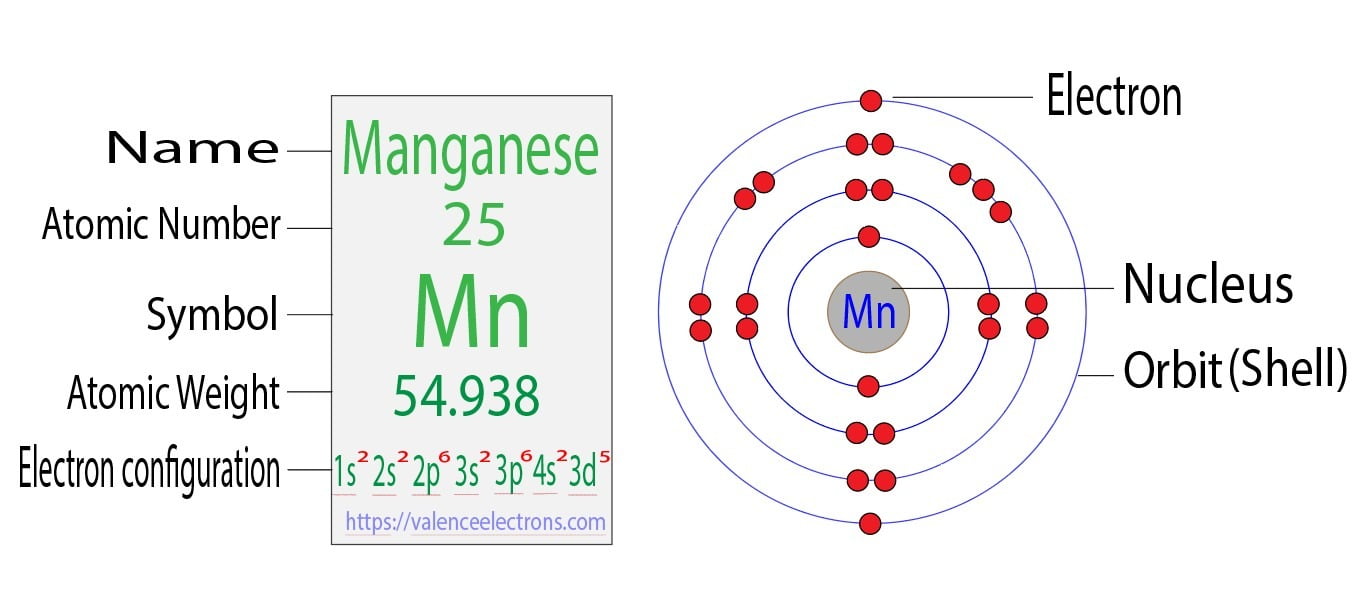
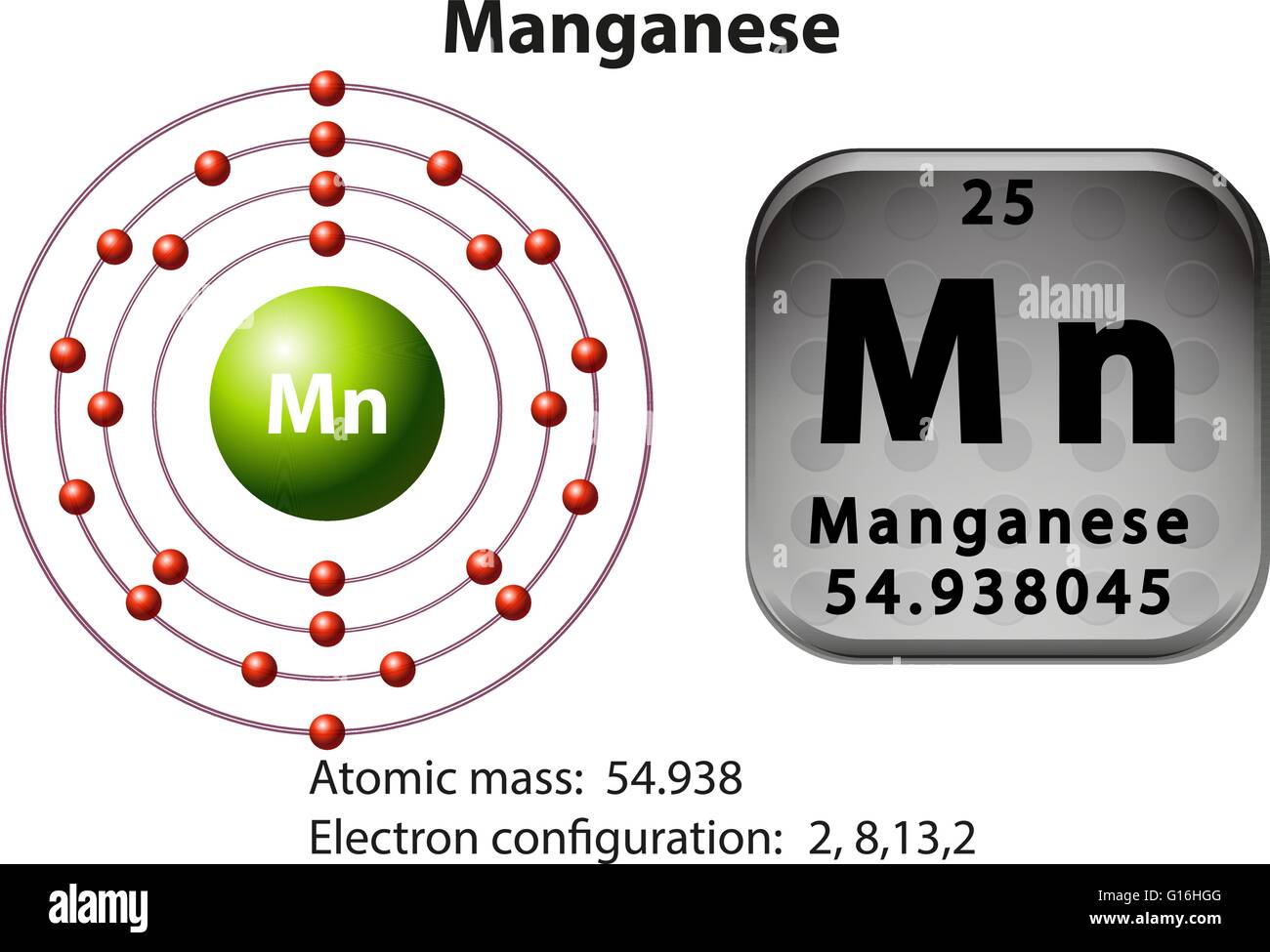
Write The Complete Electron Configuration For The Manganese Atom
So, let's talk about electrons. Yeah, I know, riveting stuff. But sometimes, you just gotta get it out there, right? Like that one song you can't stop humming, or that questionable fashion choice you made in middle school. This is my “questionable fashion choice” in the world of science. It’s about figuring out where all the tiny little bits of electricity hang out in an atom. And today, our special guest star is the mighty Manganese atom. Go, Manganese, go!
Now, you might think writing out an electron configuration is like organizing your sock drawer. A necessary evil, maybe. But I think it’s more like crafting a tiny, cosmic address for each electron. It’s a way of saying, "Okay, little guy, you live here, in this specific orbital. Don't wander off!" And for Manganese, it’s quite the sprawling neighborhood. It’s like trying to fit all your friends into a studio apartment. Things get cozy.
Let’s dive in, shall we? We start from the very beginning, with the smallest, closest shells. Think of it like checking into a fancy hotel, room by room. First up, we have the 1s orbital. It’s like the lobby, where the first few electrons get to hang out. They’re super close to the nucleus, feeling all the positive vibes. For Manganese, we’ve got two electrons chilling in the 1s. So, that’s 1s². Easy peasy, right? Almost too easy. It makes you wonder what’s coming next.
Then, we move on to the next floor, the second shell. This one has a bit more space. We’ve got another s orbital, the 2s. It’s like the next best room, still nice and central. Two more electrons plop down here. So, we add 2s² to our growing address. But wait, there’s more! On this second floor, we also have the p orbitals. Think of these as the slightly fancier suites, with multiple connected rooms. They can hold a total of six electrons. And you guessed it, Manganese fills those up too. So, we’re looking at 2p⁶. Our address is getting longer. It's like we're adding more zip codes to our mailing list.
Now, we ascend to the third shell. This is where things start to get interesting, and maybe a little crowded. We’ve got the 3s orbital, which takes another two electrons: 3s². Still pretty straightforward. But then, like a really popular coffee shop, the 3p orbitals are bustling with six electrons: 3p⁶. Our electron address is really starting to look like a novel now. It’s getting so long, you might need a bookmark just to keep track of it all.
And then, we hit the grand finale, the masterpiece, the part that makes me chuckle a little. The 3d orbitals. These are the ultra-luxury penthouses. They can hold up to ten electrons. And Manganese, bless its electron-filled heart, has five of them hanging out in the 3d orbitals. It’s like that one friend who brings way too many snacks to a party. So, that’s 3d⁵. It's not quite full, but it's got a good crowd. It’s like a lively get-together, not a packed sardine can, but definitely not an empty ballroom either.
So, if we put it all together, the complete electron configuration for Manganese looks like this: 1s²2s²2p⁶3s²3p⁶3d⁵. Wait, did I forget something? Oh, yes! The very last electrons, the ones that venture furthest from the nucleus. These are in the fourth shell. We’ve got the 4s orbital, which can hold two electrons. For Manganese, it has two in there. So, 4s². But here’s the funny part. Sometimes, depending on how you’re feeling, or what the atom is up to, the order gets a little mixed. It’s like rearranging furniture in your house – it looks different but it’s still the same stuff. The standard way we usually write it, to show how things are filled, is to put the 4s electrons after the 3d ones. So, if we’re being proper, it’s actually 1s²2s²2p⁶3s²3p⁶4s²3d⁵. It’s like the shy guest who only comes out once everyone else has settled in.

My unpopular opinion? Electron configurations are like the ultimate procrastination tool. You can spend hours perfectly arranging these tiny particles in your head, feeling incredibly productive, when in reality, you're just… thinking about electrons. And isn't that just the most delightful way to avoid doing laundry?
But you know, there's a certain elegance to it, isn't there? This intricate dance of electrons, each with its own address. It’s like a tiny, invisible city within every atom. And Manganese, with its 1s²2s²2p⁶3s²3p⁶4s²3d⁵ configuration, is a bustling metropolis. It’s got all the amenities, all the different neighborhoods. It’s living its best electron life.
So next time you encounter the mighty Manganese, whether it’s in a battery or a supplement, give a little nod to its electron configuration. It’s a testament to its atomic personality. And maybe, just maybe, you’ll crack a smile at the sheer, delightful absurdity of it all. Because, let’s be honest, talking about electrons is way more fun when you’re imagining them as tiny little roommates in a very, very strange apartment building. You know, the one governed by the laws of quantum mechanics. Which, frankly, sounds like a nightmare to furnish.
