Write Equation For The First Ionization Energy Of Neon
Hey there, ever found yourself staring at a bottle of neon lights and wondering, "What's the deal with that glow?" Well, get ready for a little chat about something called the first ionization energy, and specifically, why neon is such a superstar when it comes to this. It sounds super science-y, right? Like something you'd only find in a dusty old textbook. But trust me, it's actually pretty cool and can help us understand a bunch of stuff, even about things we see every day!
So, let's break it down. Imagine an atom, the tiny building blocks of everything around us. Think of it like a miniature solar system. In the center, you have the nucleus, which is like the sun. And buzzing around it are electrons, like tiny planets. These electrons are what make things happen in the world of chemistry. They’re the ones who decide if atoms want to hold hands, share their toys (electrons, that is!), or just do their own thing.
Now, the first ionization energy is basically the energy it takes to nudge one of those electrons away from its atom. Think of it like trying to convince a really comfy cat to get off your favorite armchair. It takes a certain amount of persuasion, a bit of effort, to get that little guy to budge. For some atoms, their electrons are super tightly held, like a toddler clinging to a parent's leg at the playground. It takes a ton of energy to pull them away. For others, their electrons are more like a friendly dog who's happy to come over for a treat. A little bit of encouragement, and poof, they're out.
And this is where our friend, neon, struts onto the stage. Neon, the stuff that makes those vibrant red signs glow so beautifully, is actually kind of a loner. Not in a sad way, but in a very content, self-sufficient way. Atoms, like people, have a deep-seated desire to be stable. They like having their electron orbits neatly filled, like having all your socks paired up in the drawer. It makes them feel complete, you know?
Neon, with its 10 electrons, has its electron "orbits" perfectly arranged. It's got its inner shell filled up, and its outer shell is also perfectly complete. It's like it's already won the atomic lottery and doesn't need any extra electrons or feel the urge to give any away. This makes neon atoms incredibly stable and, frankly, a bit resistant to change. They're perfectly happy just the way they are.
So, when we talk about the equation for the first ionization energy of neon, we're essentially describing the energy needed to overcome this happy, stable state and yank just one electron away. It's like trying to convince that perfectly content cat, who's just discovered the sunbeam, to get up and go for a walk. You're going to need to offer something pretty darn enticing, or just have a lot of patience and persistence.
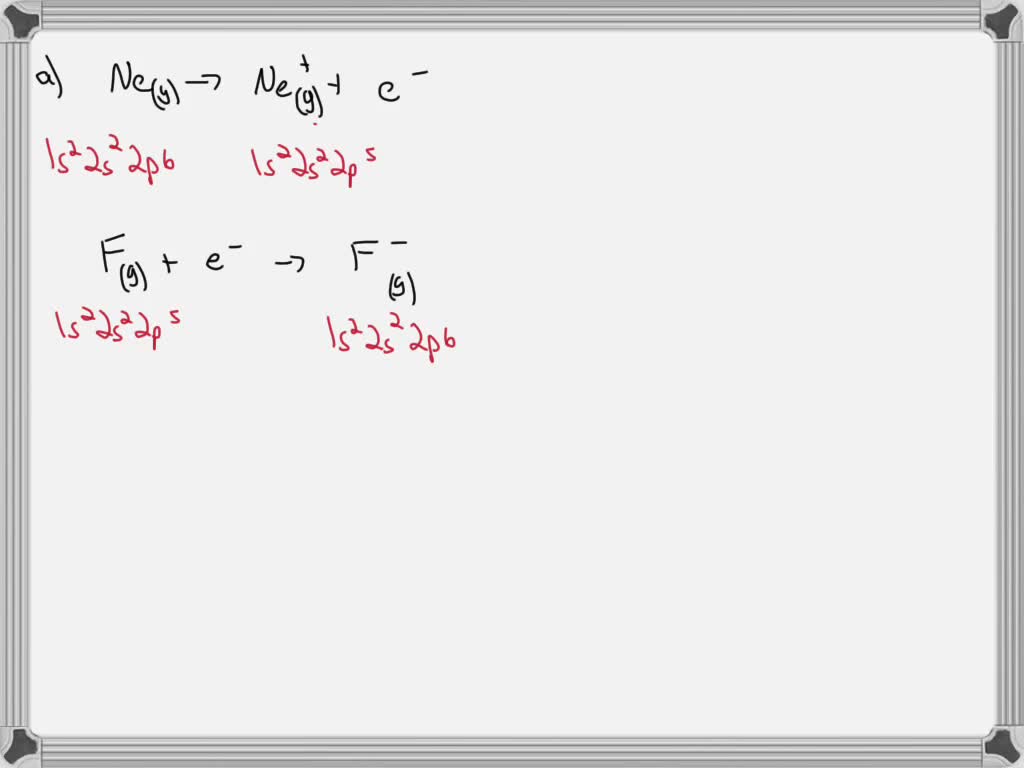
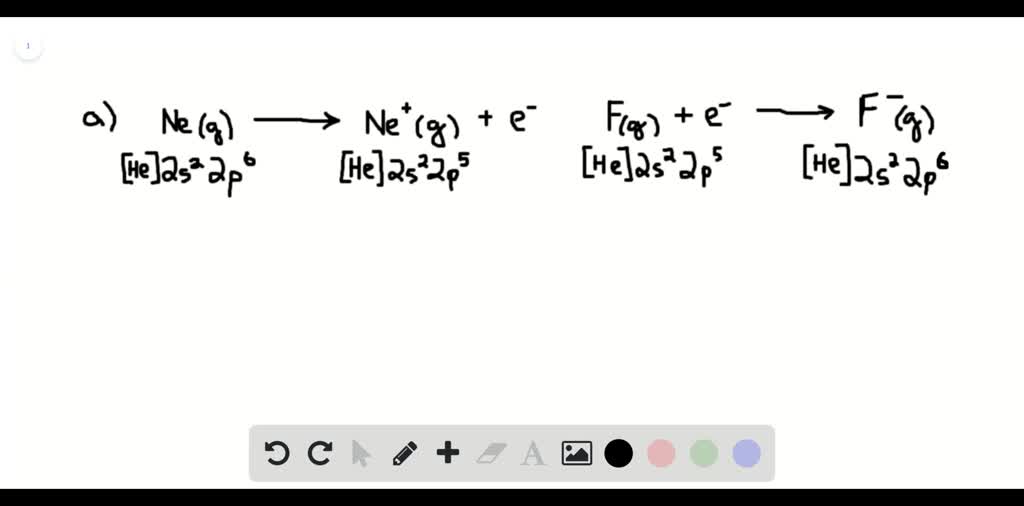
The "equation" itself isn't like a math problem you solve with numbers in the traditional sense, at least not for a general audience. It's more of a concept, a representation of a process. We can write it out like this, using chemical symbols:

Ne (g) + Energy → Ne+ (g) + e-
Let's decode this little bit of magic.
- Ne (g): This is our friend, neon, in its gaseous state. Think of it as a single, happy neon atom floating around.
- + Energy: This is the effort we're putting in. It's the "oomph" required to do the deed.
- →: This is our arrow, indicating that a change is happening, a transformation.
- Ne+ (g): This is what we get after we've successfully persuaded one electron to leave. It's a neon ion, carrying a positive charge because it's lost a negatively charged electron. Think of it as the neon atom looking a little bit lighter and with a slight air of surprise.
- + e-: And this, my friends, is the electron that we've managed to liberate. It's now free to roam!
What's really neat about neon is that the energy required for this process is very high. Much higher than for many other elements. Why? Because, as we talked about, neon is already so darn satisfied with its electron arrangement. It's like trying to steal the last cookie from someone who’s already had a whole plate. They’re going to guard it fiercely!
This high ionization energy is why neon doesn't readily form chemical bonds. It's not looking to share, it's not looking to gain. It's perfectly content in its noble gas state. And this stability is why it’s used in those iconic signs. It doesn’t react with the air or the glass, it just sits there, glowing gloriously when electricity zaps it, releasing that signature color. It’s the reliable friend of the periodic table, always there, always itself.
So, why should you care about the first ionization energy of neon? Well, it helps us understand the fundamental behavior of elements. It explains why certain elements are super reactive (like sodium, which is eager to give away an electron) and why others, like neon, are so inert (they don't do much!). This impacts everything from how we make medicines to how we create new materials. It’s the tiny invisible dance of electrons that dictates so much of our visible world.

Think about it. If all elements were as unreactive as neon, we wouldn't have water, or plants, or even ourselves! Chemical reactions are all about atoms sharing, gaining, and losing electrons. Neon, with its high ionization energy, is the exception that proves the rule, the calm center in a bustling universe of chemical transformations. It’s the quiet observer, perfectly content in its own atomic bubble, and that very contentment dictates its place and behavior in the grand scheme of things.
Next time you see a neon sign, instead of just seeing the pretty glow, you can think about the incredible stability of those neon atoms. You can marvel at the energy it takes to even think about disturbing their perfect electron arrangement. It’s a little peek into the fundamental forces that shape our universe, all thanks to the noble gas that just wants to be left alone, but in doing so, lights up our world.
And that, my friends, is the fun and accessible story behind the first ionization energy of neon. It’s not just a scientific concept; it's a testament to the beauty of stability and the unique personalities of the tiny building blocks that make up everything we know and love. Pretty cool, right?
