Write A Chemical Equation That Illustrates The Autoionization Of Water

Hey there, ever stop and think about water? I mean, really think about it? It’s everywhere, right? From that morning cup of coffee that kicks off your day to the giant ocean that makes you feel tiny and insignificant (in a good way, usually). We’re practically made of it, and our planet is a big ol' blue marble because of it. But have you ever considered that even this seemingly simple stuff, this H2O we take for granted, is secretly a bit of a drama queen?
Yeah, I know, sounds a bit dramatic, doesn't it? Water? Drama? But stick with me here. It turns out water isn't just chilling out, being all wet and predictable. It’s got this inner life, a tiny, almost imperceptible hustle going on behind the scenes. And the best part? You don't need a chemistry degree to get it. Think of it like this: you know how sometimes you’re just minding your own business, and suddenly you’re a little bit tired, or a little bit excited? Water’s got that vibe, but on a molecular level.
So, what's the big deal? Well, it’s something called autoionization. Fancy word, I know. Sounds like something a robot would do before it goes on strike. But really, it’s just water being a bit…generous. Or maybe a bit needy. It’s like water is giving away little pieces of itself, and then, like a boomerang, it gets a piece back. It’s a constant, subtle exchange. A molecular dance, if you will.
Imagine you’ve got a bunch of perfectly happy water molecules, just bobbing along. They’re all cozy, two hydrogen atoms holding hands with an oxygen atom – that's our good ol' H2O. They’re like a stable little family unit. But, every now and then, one of these water molecules is feeling a little… unhinged. It’s like it’s got an extra toy it doesn't know what to do with, or maybe it's just feeling a bit lonely for some company.
So, what does it do? It does something pretty wild. It splits up. Not in a messy divorce way, thankfully. More like a temporary separation with the goal of creating something new. One of the hydrogen atoms, which is basically just a positively charged little dude (we call it a proton or, in this context, a hydronium ion precursor), decides to ditch its oxygen buddy for a bit.
This little rogue hydrogen then goes looking for a new home. And where's the most welcoming place for a lonely, positively charged hydrogen? Another, nearby water molecule, of course! It’s like that friend who always needs a place to crash. This new water molecule, the one that’s just been accosted by a stray proton, is now a bit overwhelmed. It's got an extra hydrogen, making it H3O. This is our hydronium ion. Think of it as a water molecule that’s just been given a surprise extra sidekick. It's a bit crowded, but it's also quite stable, happily carrying that extra positive charge.
But wait, there's more! When that hydrogen atom detached from its original water molecule, it left behind something. It left behind an oxygen atom with an extra electron. This is now a negatively charged entity. We call this the hydroxide ion. So, our water molecule, which was all H2O, has now transformed into a positively charged hydronium ion (H3O+) and a negatively charged hydroxide ion (OH-). It’s like a molecule had a baby and a solo parent simultaneously!
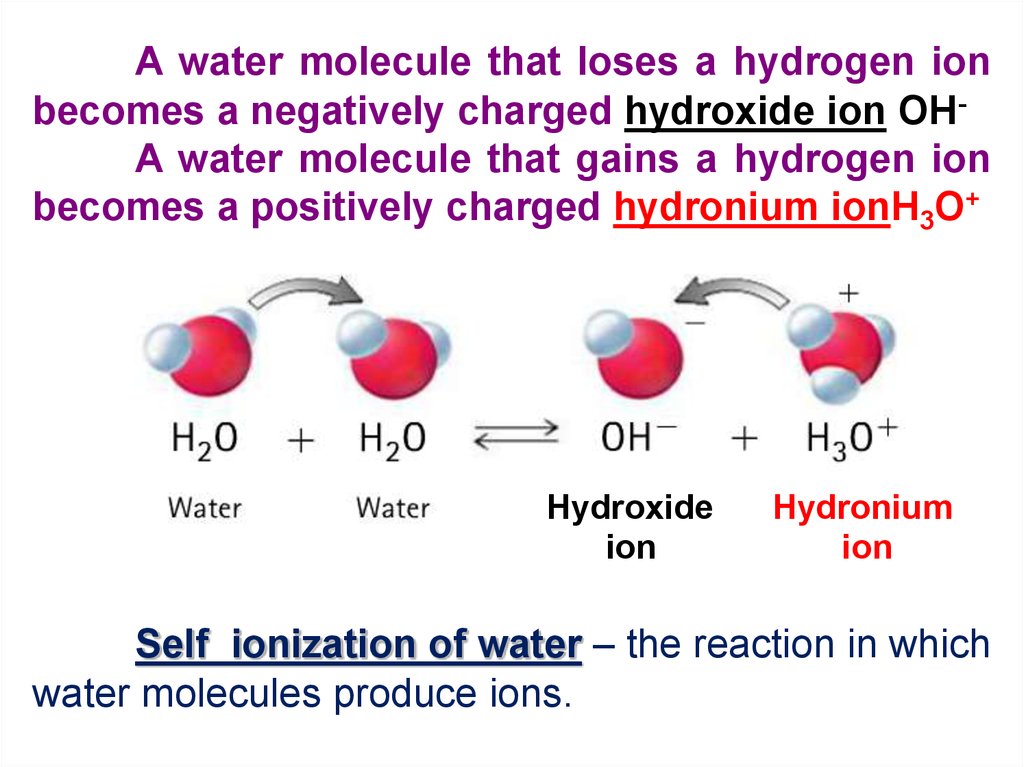
This whole process, this splitting and reforming, is the autoionization of water. It's literally water ionizing itself. Pretty neat, right? It doesn't need any outside help, no fancy catalysts, no encouraging words from other substances. Water is a self-starter when it comes to this little bit of molecular drama.
So, the chemical equation that describes this little shindig looks like this:
H2O ⇌ H+ + OH-
Now, before you go thinking I'm pulling a fast one, let me explain that little squiggle in the middle. That double arrow (⇌) is super important. It’s not a one-way street, my friends. It means that this is an equilibrium reaction. It’s like a seesaw. Water molecules are splitting up into ions, and at the exact same time, those ions are getting back together to form water molecules again. It’s a constant back-and-forth, a never-ending molecular disco.
Most of the time, water is just happy being H2O. The vast majority of water molecules are just… water molecules. They’re not all breaking up and forming ions. It's a tiny percentage. Think of it like the quiet people in a crowded room. They’re there, but they’re not making a fuss. But even that tiny percentage is significant enough to make a difference in how water behaves, especially when it comes to things like acidity and alkalinity.
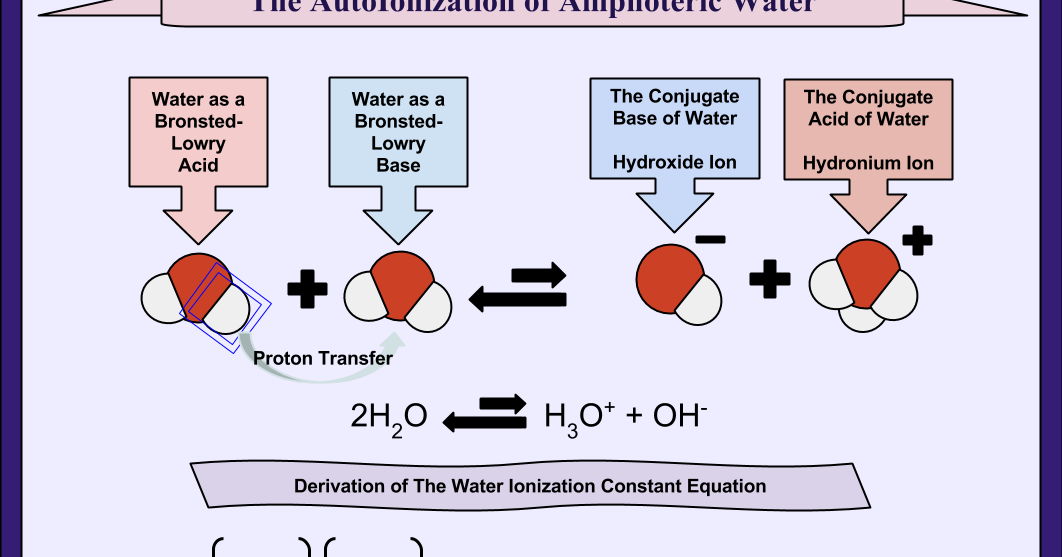
Let's revisit that H+. While we write it as just "H+" in the simplified equation, in reality, that lone proton is way too eager and way too positive to exist by itself for long. It’s like a toddler with a sugar rush, bouncing off the walls. So, as we mentioned before, it immediately latches onto another water molecule.
This is where the more detailed, and some might say more accurate, representation of water's autoionization comes in. Instead of just H+, we usually represent it as H3O+. So, the equation looks a bit more like this:
2H2O ⇌ H3O+ + OH-

See? We’ve got two water molecules involved now. One of them is the one that gets all dramatic and splits, and the other one is the one that becomes the welcoming party for the stray proton. This second equation is often preferred because it paints a more complete picture of what’s actually happening in pure water.
Think of it like this: you’re making a sandwich. You’ve got your bread (water molecule 1). You take out the filling (the proton). Now you have empty bread (the hydroxide ion) and a filling that needs to go somewhere. You grab another piece of bread (water molecule 2) and put the filling in it. Now you have a new sandwich with extra filling (the hydronium ion) and a lonely piece of empty bread (the hydroxide ion). And then, the filling might pop out again and find a new sandwich, and the empty bread might get reattached to some filling.
This whole process is happening in every drop of water you’ve ever encountered. In your tap water, in your bottled water, even in that tiny speck of dew on a spiderweb. It’s this constant, silent molecular makeover. It’s why water is such a great solvent, able to dissolve so many different things. It’s because of these little charged particles, the hydronium and hydroxide ions, that are always zipping around.
The concentration of these ions is incredibly low. Like, really low. For every billion water molecules, only about two of them have decided to engage in this ionization drama. It’s a statistical anomaly, a molecular fluke. But these tiny numbers are the backbone of what we understand as pH. You know, that scale that tells you if something is acidic, like lemon juice (low pH, more H+), or basic, like baking soda (high pH, more OH-). Even though the concentration is minuscule, the balance between H3O+ and OH- is what dictates that balance.

In pure water, the concentration of hydronium ions (H3O+) is exactly equal to the concentration of hydroxide ions (OH-). This perfect balance is what makes pure water neutral. It’s neither acidic nor basic. It’s just… water. Calm, collected, and perfectly balanced. It’s like a perfectly aligned yoga pose, but with molecules.
Now, when you add something to water, like a splash of vinegar (which is acidic), you’re essentially tipping the scales. You’re adding more H+ (or rather, H3O+) to the mix. This disrupts the delicate balance. The water molecules are still autoionizing, but now there’s this external force also contributing to the number of hydronium ions.
Conversely, if you add something like soap (which is basic), you’re adding more OH-. Again, the balance is thrown off. Water is constantly trying to get back to its happy equilibrium, but these additions push it in one direction or another.
It’s a constant negotiation between water’s innate tendency to autoionize and whatever else you decide to throw into the mix. And it’s this constant, almost invisible, molecular negotiation that makes water so fundamental to life and to so many chemical reactions.
So, next time you’re sipping on a glass of water, or washing your hands, or even just looking at a puddle, remember the tiny drama queen that resides within. Remember the H2O molecules having their little molecular breakdowns and makeovers. It’s a quiet, constant process, but it’s a testament to the fact that even the most common things can have hidden depths and fascinating inner lives. And that, my friends, is pretty darn cool.
