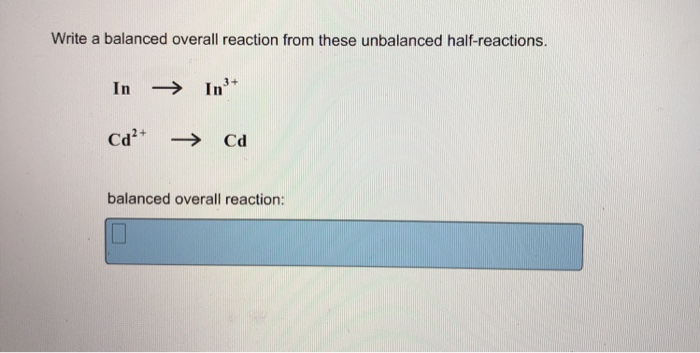
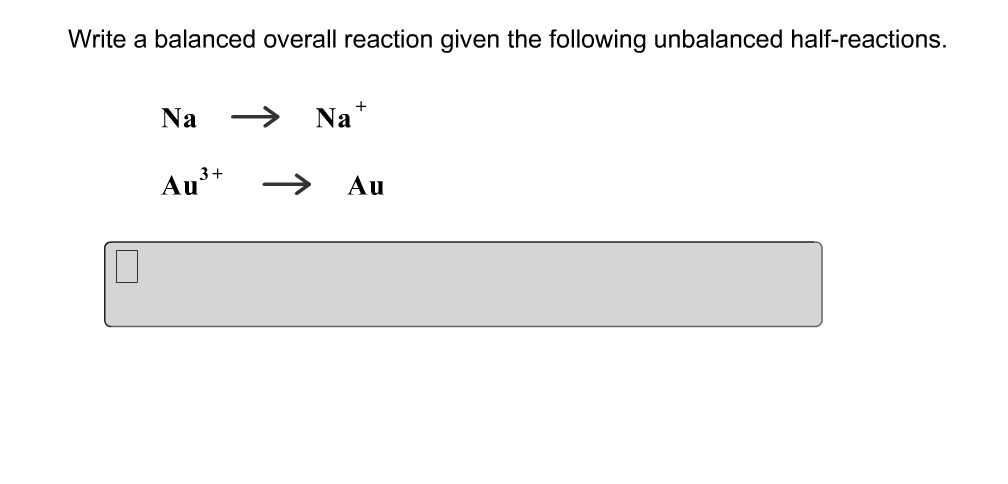
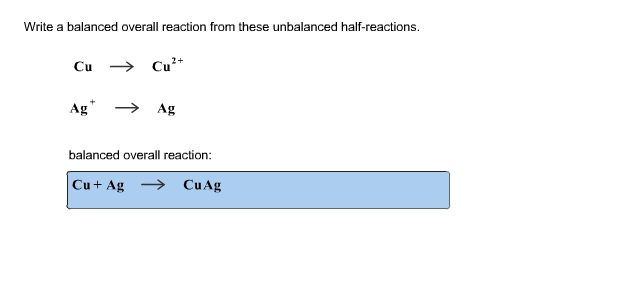
Write A Balanced Overall Reaction From These Unbalanced Half Reactions

Hey there! So, you're diving into the wild world of chemistry, huh? Fancy a coffee while we tackle some equations? Grab your mug, because we're about to become chemical reaction superheroes. Seriously, it's not as scary as it sounds. Think of it like putting together a really complicated puzzle, but instead of a picture of a fluffy cat, we're building… well, stuff!
Today's mission, should you choose to accept it (and you totally should!), is figuring out how to write a balanced overall reaction from some unbalanced half reactions. Sounds a bit like science jargon, right? But stick with me. It's like taking two separate stories and weaving them into one epic saga.
So, what even are these half reactions? Basically, they're like the individual acts in a play. One half reaction shows what's happening with the oxidation (losing electrons, like giving away your favorite snacks), and the other shows what's happening with the reduction (gaining electrons, like snagging the last cookie). They always happen together, like best buds, but sometimes the chemist writing them down splits them up for clarity. It’s like a chef preparing the appetizer and the main course separately before bringing them to your table. Makes sense?
Our goal, my friend, is to smoosh these two halves together and make sure everything balances out. Like, you can't just magic electrons out of thin air, right? Or have them disappear into the void. That's a big no-no in chemistry. Everything has to be accounted for. Think of it as the universe’s ultimate balancing act.
Let's dive into an example, shall we? Because seeing is believing, and doing is even better! Imagine we have these two beautifully, awesomely unbalanced half reactions:
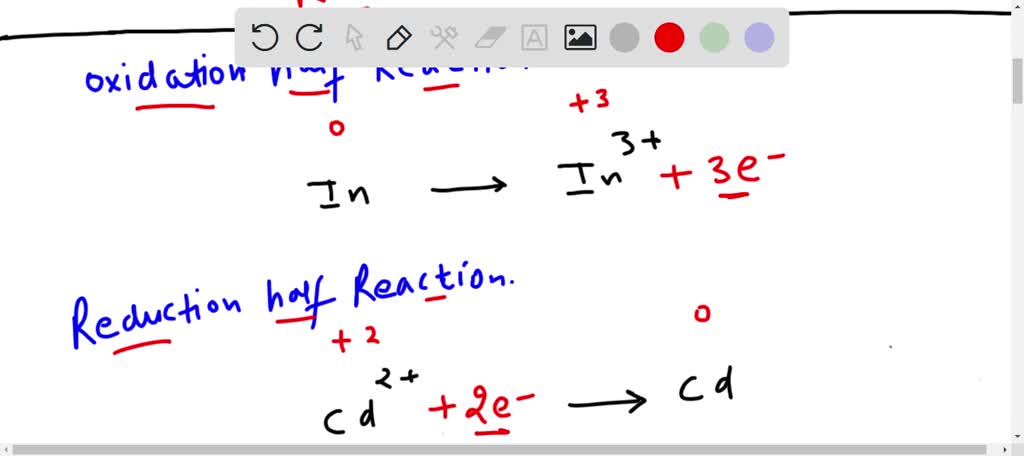
Half Reaction 1 (Oxidation):
Zn(s) → Zn2+(aq) + 2e-
Half Reaction 2 (Reduction):
Cu2+(aq) + 2e- → Cu(s)
Now, take a peek. See the e-? Those are our electrons. In the first one, zinc (Zn) is giving away two electrons, becoming a positively charged ion. Hence, oxidation! It's like that friend who’s always lending you money… and never asking for it back. Generous, right?
In the second one, copper ions (Cu2+) are grabbing those two electrons and becoming solid copper. Reduction! This one’s like the friend who’s always borrowing your charger and actually plugging it in. Productive!
So, the first step is to look at the number of electrons in each half reaction. Notice anything? In our example, we have 2e- on the product side of the first reaction, and 2e- on the reactant side of the second reaction. It's a perfect match! It's like they were made for each other, these two half reactions. The universe is already smiling on us.
What if they don't match? Oh, that's where the real fun begins! Imagine one half reaction is handing out two cookies, and the other is only willing to accept one. What do you do? You gotta make them equal, right? You'd have to, like, multiply the second half reaction by two, so it’s ready to accept four cookies. Or maybe the first reaction is giving away a whole pizza, and the second only wants a slice. You’d have to bake more pizzas! This is where we bring in the magic of multiplication.
Let's pretend, just for a sec, that our reduction half reaction looked like this instead:
Hypothetical Half Reaction 2 (Reduction):
Cu2+(aq) + 1e- → Cu(s)
See? Only one electron is involved here. But our first half reaction is still happily giving away two. So, what do we do? We need to get the electrons to be equal. We've got a 2 on one side and a 1 on the other. The easiest way to make them equal is to multiply the second reaction by 2. So, it would become:
Adjusted Hypothetical Half Reaction 2:
2[Cu2+(aq) + 1e- → Cu(s)]
Which then becomes:
2Cu2+(aq) + 2e- → 2Cu(s)
Now, both half reactions are dealing with two electrons! See how that works? It's all about finding the least common multiple of the electrons. Think of it like getting your chores done equally. If one sibling has to do 2 dishes and the other has to do 1, you'd tell them to do 2 sets of dishes each, so it's fair. Or something. My sibling negotiation skills are rusty.
Okay, back to our original, beautifully balanced example where the electrons already matched!
Zn(s) → Zn2+(aq) + 2e-
Cu2+(aq) + 2e- → Cu(s)
Now that our electrons are like a perfectly matched pair of socks, we can combine these two half reactions. We just add everything up from the reactant sides and everything up from the product sides. It's like gathering all your ingredients before you start cooking. You wouldn't just randomly toss things into the pot, would you? Probably not. Unless you're feeling really adventurous, and then maybe you should stick to puzzles.
So, let's do it. On the reactant side, we have Zn(s) and Cu2+(aq) and 2e-. On the product side, we have Zn2+(aq) and 2e- and Cu(s).
Putting it all together, we get:
Zn(s) + Cu2+(aq) + 2e- → Zn2+(aq) + 2e- + Cu(s)
But wait! Remember those electrons? They're like the ghost in the machine. They were transferred, yes, but they aren't part of the final product or the initial reactants in the overall reaction. They just facilitated the exchange. So, just like you'd remove any stray sprinkles that fell off your cookie onto the counter, we cancel out the electrons from both sides.
Poof! Gone. Vanished into the chemical ether. Because they are on both sides of the arrow, we can subtract them from each side. It's like if you had 5 apples on one side of a scale and 3 apples plus 2 pears on the other. If you take away 2 apples from each side, you still have 3 apples on one side and 2 pears on the other. See?
So, after we wave goodbye to our electrons, what's left is our beautiful, balanced overall reaction:
The Balanced Overall Reaction:
Zn(s) + Cu2+(aq) → Zn2+(aq) + Cu(s)
Ta-da! Isn't that neat? We started with two separate stories, made sure the electron count was equal, and then merged them into one complete, balanced narrative. This tells us that solid zinc reacts with copper ions to form zinc ions and solid copper. It's the whole shebang!
Let's just do a quick check, because a good scientist always checks their work. On the reactant side, we have one zinc atom and one copper ion. On the product side, we have one zinc ion and one copper atom. And the charges? On the reactant side, we have a neutral zinc (0 charge) and a +2 copper ion. Total charge: +2. On the product side, we have a +2 zinc ion and a neutral copper atom (0 charge). Total charge: +2. Perfectly balanced. Even the charges are happy campers!
This is actually a pretty common reaction you might see in batteries or electroplating. So, not only are you learning chemistry, you're also learning how the world around you works. Pretty cool, right?
Now, what if the reactions are a little more… complicated? Like, what if they're happening in an acidic or basic solution? Don't panic! It just means we might have to do a little extra work to balance the oxygen and hydrogen atoms. This is where things can get a tiny bit fiddly, but it’s still totally manageable.
If it's an acidic solution, we can add H2O molecules to balance oxygen and H+ ions to balance hydrogen. Think of it like adding extra water to your tea if it's too strong, and a pinch of sugar if it’s too weak. You adjust until it’s just right!
If it's a basic solution, it's similar, but a little trickier. We add H2O to balance oxygen and hydrogen, but instead of adding OH- directly, we often add H+ first to balance the hydrogens, and then we convert those H+ ions into OH- by adding OH- to both sides of the equation. Confusing? A little. But the H+ and OH- will combine to form H2O, which we can then cancel out if it appears on both sides. It's like a chemical magic trick!
Let’s say we have an acidic solution and our half reactions involve oxygen. For example, maybe one half reaction is:
MnO4-(aq) → Mn2+(aq)
See the oxygen? We need to balance that. We can add H2O to the side that needs oxygen. Since the right side doesn't have any oxygen, we'd add 4H2O to the right:
MnO4-(aq) → Mn2+(aq) + 4H2O(l)
Now, we need to balance the hydrogens. We have 8 hydrogens on the right (from the 4H2O), so we need 8 hydrogens on the left. In an acidic solution, we add H+ ions:
8H+(aq) + MnO4-(aq) → Mn2+(aq) + 4H2O(l)
And finally, we balance the charge. On the left, we have 8 positive charges from H+ and a -1 charge from MnO4-, for a total of +7. On the right, we have a +2 charge from Mn2+ and 0 from H2O. To make the charges equal, we need to add 5 electrons (5e-) to the left side:
8H+(aq) + MnO4-(aq) + 5e- → Mn2+(aq) + 4H2O(l)
Phew! That's one balanced half reaction! And that's just ONE half. Imagine doing that for two! But honestly, once you get the hang of it, it becomes almost second nature. Like riding a bike, but with more equations and less scraped knees.
So, the general steps, my friend, are:
- Separate the overall reaction into its oxidation and reduction half reactions. (Usually given to you, thank goodness!)
- Balance all atoms except oxygen and hydrogen in each half reaction.
- Balance oxygen atoms by adding
H2Omolecules to the side that needs oxygen. - Balance hydrogen atoms by adding
H+ions to the side that needs hydrogen (for acidic solutions). - Balance the charge in each half reaction by adding electrons (
e-) to the appropriate side. - If you have a basic solution, you do the above steps, then multiply everything by
OH-to convertH+toH2Oand balance the extraOH-. - Multiply one or both half reactions by integers so that the number of electrons lost in oxidation equals the number of electrons gained in reduction.
- Add the two balanced half reactions together.
- Cancel out any species that appear on both sides of the overall reaction (like electrons!).
- Check that the atoms and charges are balanced on both sides.
It might seem like a lot of steps, but trust me, each one is a little victory. And the feeling of finally getting that perfectly balanced equation? Priceless. It's like solving a riddle and getting a standing ovation from your textbook.
So, next time you see those unbalanced half reactions staring you down, don't get intimidated. Grab your metaphorical coffee, take a deep breath, and channel your inner chemistry guru. You've got this. And hey, if all else fails, just remember: it's all about making sure those electrons behave themselves!
