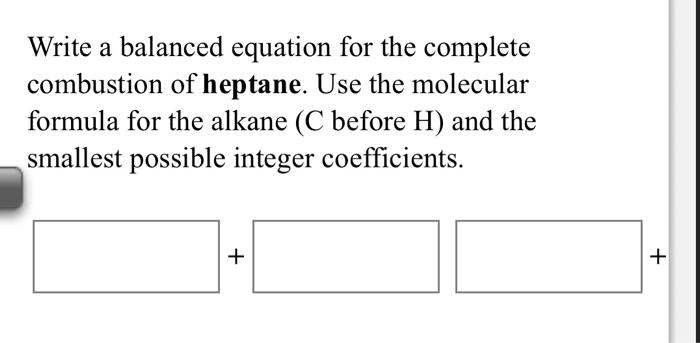
Write A Balanced Equation For The Complete Combustion Of Heptane
Ever wondered about the secret life of fuel? We're talking about the stuff that powers our cars and makes our campfires roar! Today, we're diving into the exciting world of heptane combustion. It’s like a tiny, explosive dance that fuels our everyday adventures.
Think of heptane as a tiny energy package. It’s a kind of hydrocarbon, which basically means it's made of hydrogen and carbon atoms all linked together. When these little packages get together with oxygen, magic happens. It’s a full-on party, and everyone’s invited!
This isn't just any old burning. We're talking about complete combustion. This means all the fuel gets used up, and we get the most energy out of it. It’s like getting a perfect score on a test! No wasted energy, just pure power.
So, what does this spectacular show look like on paper? It’s all about balance. We need to make sure we have the right amount of everything on both sides of the reaction. Think of it like a recipe: too much or too little of an ingredient can ruin the dish.
Let’s meet our main ingredient: heptane. Its chemical formula is C7H16. This tells us that each molecule of heptane has 7 carbon atoms and 16 hydrogen atoms. They’re all holding hands, ready for their big moment.
Next up, the crucial partner: oxygen. We need lots of it for this party to be complete. Oxygen’s chemical formula is simple: O2. It’s the stuff we breathe, but in this context, it’s the fuel for the fire.
When heptane and oxygen get together, they put on a dazzling display. They break apart their bonds and rearrange themselves into something new and exciting. It’s a chemical makeover, and the results are stunning.
The main stars of the show are carbon dioxide and water. You know, the stuff we exhale and the liquid that keeps us alive. Carbon dioxide’s formula is CO2, and water’s is H2O. They are the happy byproducts of this energetic reaction.

But here’s where the fun really begins: balancing the equation. We can't just say "heptane plus oxygen makes carbon dioxide and water." We need to be precise. It's like being a chemist DJ, mixing the perfect beat.
Our starting point is: C7H16 + O2 → CO2 + H2O. This is the raw idea, but it’s not balanced yet. We have 7 carbons on the left, but only 1 on the right. That's a mismatch!
To fix the carbon count, we need to add a coefficient in front of the carbon dioxide. Since we have 7 carbons in heptane, we need 7 molecules of CO2. So, our equation starts to look like this: C7H16 + O2 → 7CO2 + H2O. Getting closer!
Now, let's look at the hydrogen. We have 16 hydrogen atoms in heptane. On the right side, each molecule of water (H2O) only has 2 hydrogen atoms. To get 16 hydrogen atoms, we need 8 molecules of H2O. So, we add an 8 in front of the water: C7H16 + O2 → 7CO2 + 8H2O. The elements are starting to line up!
We’ve balanced the carbon and hydrogen. Now, for the tricky part: oxygen. This is where things get a little more involved. On the right side of our equation, we have 7CO2, which means 7 * 2 = 14 oxygen atoms from the carbon dioxide. And we have 8H2O, which means 8 * 1 = 8 oxygen atoms from the water.
Add those up: 14 + 8 = 22 oxygen atoms in total on the product side. So, we need 22 oxygen atoms on the reactant side, coming from oxygen molecules (O2). Since each O2 molecule has 2 oxygen atoms, we need 11 molecules of O2. That means we put an 11 in front of the oxygen: C7H16 + 11O2 → 7CO2 + 8H2O.
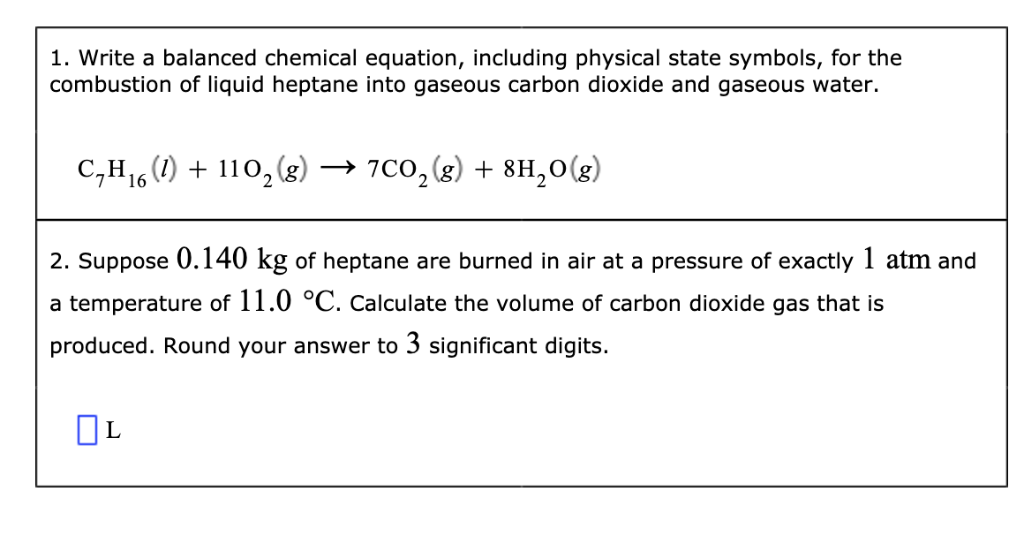
And there you have it! The balanced equation for the complete combustion of heptane. It's a beautiful, orderly arrangement of atoms. Each side has exactly the same number of carbons, hydrogens, and oxygens. It’s a chemical masterpiece.
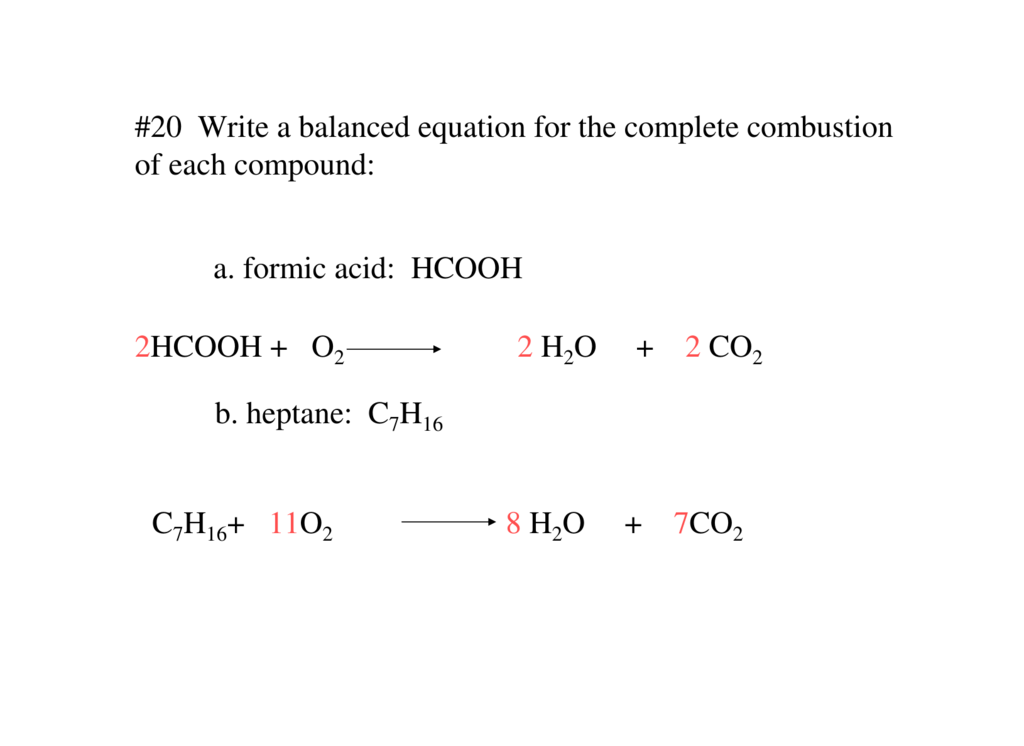
C7H16 + 11O2 → 7CO2 + 8H2O
This equation tells a story of transformation. It shows how one substance breaks down and reforms into entirely new ones, releasing energy in the process. It’s the essence of fire, the power that drives so much of our world.
Why is this so entertaining? Because it’s a peek behind the curtain of everyday phenomena. It’s the invisible magic that makes your car zoom or your stove heat up. Understanding it is like unlocking a secret code.
The beauty lies in its simplicity and its complexity. On the surface, it’s just chemicals reacting. But beneath that, it’s a precise, predictable dance of atoms governed by fundamental laws. It’s elegant and powerful.
This isn't just for scientists in lab coats. This knowledge is for everyone who’s ever wondered "how does that work?" It's about demystifying the world around us. And it’s surprisingly fun!
The balanced equation is like a perfectly choreographed ballet. Every dancer (atom) has its role, and every step is accounted for. Nothing is out of place, and the final result is a powerful performance.
Imagine the heptane molecule as a tiny, tightly wound spring of energy. When it meets the eager embrace of oxygen, it uncoils with a burst of heat and light. It’s a controlled explosion, a miniature star being born and dying in an instant.
The products, carbon dioxide and water, are the whispers left behind after the grand performance. They are the evidence of the energy released, the tangible proof of the chemical transformation. They are the calm after the storm.
What makes it special is that this seemingly simple equation represents a fundamental process that underpins our modern life. From powering our cities to cooking our meals, combustion is everywhere. And heptane combustion is a classic example.
It’s like having a secret handshake with the universe. When you understand this equation, you understand a little bit more about how the world works at its most basic level. It’s a sense of empowerment.
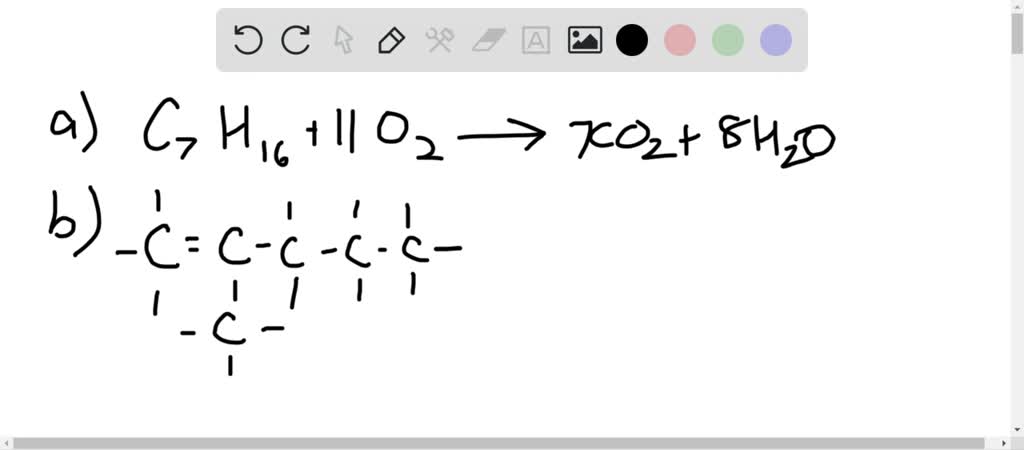
Think of the coefficients as the conductors of an orchestra. They tell us exactly how many instruments (molecules) should play their part to create the perfect harmony. Without them, the music would be chaotic.
The journey from C7H16 + O2 to 7CO2 + 8H2O is a testament to the orderliness of chemistry. It’s a reminder that even in seemingly chaotic reactions, there is a precise and predictable outcome.
So next time you see a flame or smell the exhaust from a car, you can think about this equation. You can picture those atoms dancing, transforming, and powering your world. It’s a little bit of everyday magic.
Isn't it fascinating how a few symbols can represent such a powerful and fundamental process? It's like a miniature story unfolding on the page, a story of energy and change. It's enough to make you want to grab a notebook and draw some atoms!
This is the charm of chemistry. It takes the ordinary and reveals the extraordinary. The complete combustion of heptane is just one of many incredible stories waiting to be discovered.
So, go ahead, give it a second look. Ponder the beauty of that balanced equation. It’s a gateway to understanding the world’s hidden engines, and it’s more entertaining than you might think!
