Why Does I C E Float On Water: The Real Reason + What To Do
Ever tossed a few ice cubes into a drink on a hot day and watched them bob around? It’s a small, everyday magic that most of us take for granted. But have you ever stopped to wonder why ice floats on water? It might seem like a simple question, but the answer is actually a super cool piece of science that’s not only fascinating but also has some pretty important real-world implications. Plus, understanding it can open up a whole world of fun experiments and practical knowledge!
For the absolute beginner, knowing why ice floats is like unlocking a little secret of nature. It demystifies something we see all the time and makes you feel a bit smarter. For families with curious kids, this is pure gold! It’s a fantastic jumping-off point for simple science experiments that are both educational and entertaining. Imagine the delight of your children when they understand why their ice pops don't sink to the bottom of their juice. And for the budding hobbyist, whether you're into aquariums, gardening, or even just making perfect cocktails, this knowledge can be surprisingly useful. Think about fish in a frozen pond – if ice sank, they wouldn't survive!
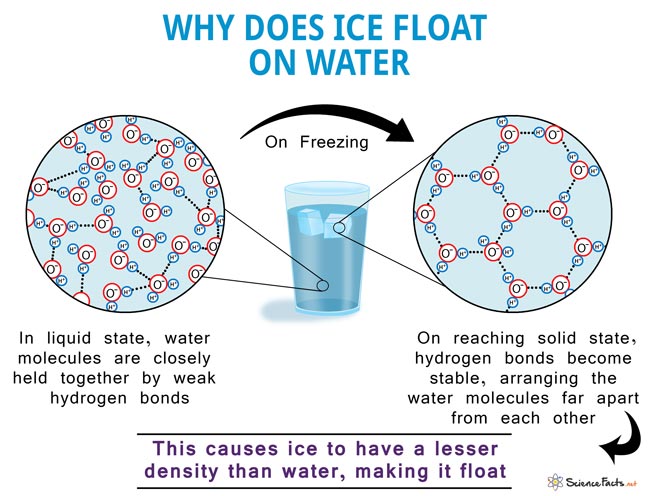
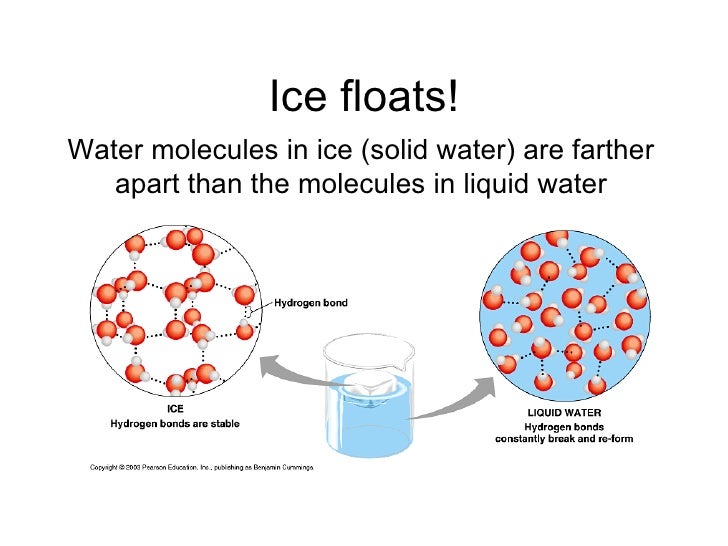
So, what's the scoop? The real reason ice floats is all about density. Density is basically how much "stuff" is packed into a certain amount of space. When water freezes into ice, its molecules arrange themselves in a specific crystal structure. This structure is a bit more spread out than when water is in its liquid state. Think of it like people in a crowded room versus people standing with their arms outstretched in a dance – the dancers take up more space. Because ice takes up more space for the same amount of water molecules, it becomes less dense than liquid water. And here’s the kicker: things that are less dense than the liquid they are in will float!
It’s a bit of an anomaly in the science world, too. Most substances become denser when they freeze. Water, however, does the opposite. This is incredibly important for life on Earth! If ice were denser and sank, lakes and oceans would freeze from the bottom up. This would kill off all the aquatic life. Instead, ice forms on the surface, acting as an insulator, protecting the water below and allowing fish and other creatures to survive through the winter. Pretty neat, huh?
Want to try a simple demonstration? Grab a glass of water and a few ice cubes. You’ll see them float. Now, try it with something else that freezes, like rubbing alcohol (though be careful with chemicals!). You’ll likely find that frozen alcohol sinks in liquid alcohol, showing you how special water's behavior is. Another fun variation is to try freezing different liquids and seeing if they float or sink in their liquid state – just be sure to do this safely and with adult supervision if needed.
Getting started with understanding this is easy. The next time you're having a cold drink, just take a moment to observe the ice. Talk about it with your kids or friends. If you want to go further, a quick search online for "density experiments for kids" will give you tons of ideas for simple, safe activities you can do at home with common household items. You can even explore how different shapes of ice might affect how they float or melt.
Ultimately, understanding why ice floats is a delightful little journey into the heart of how our world works. It’s a simple observation with profound consequences, and it proves that even the most common sights can hold a bit of everyday wonder and a whole lot of value.
