Why Are Ionic Solids Poor Conductors Of Electricity
Let's talk about something a little… electrifying. Or rather, a lack thereof. We're diving into the wonderfully dull world of ionic solids and why they're, well, a bit of a snooze when it comes to conducting electricity. I know, I know, "ionic solids" sounds like something your chemistry teacher muttered about while you were doodling. But stick with me!
Think of electricity like a really enthusiastic party. It's all about things moving around, having a grand old time, and getting from point A to point B. For electricity to flow, you need some tiny party animals – electrons – to be free to boogie. They’re the life of the party, really.
Now, imagine a bunch of these little electron party animals trapped. Not just a little bit stuck, but really, truly, deeply committed to their current location. That's pretty much the situation with ionic solids. They're like the wallflowers of the electrical world, and they’re perfectly happy staying put.
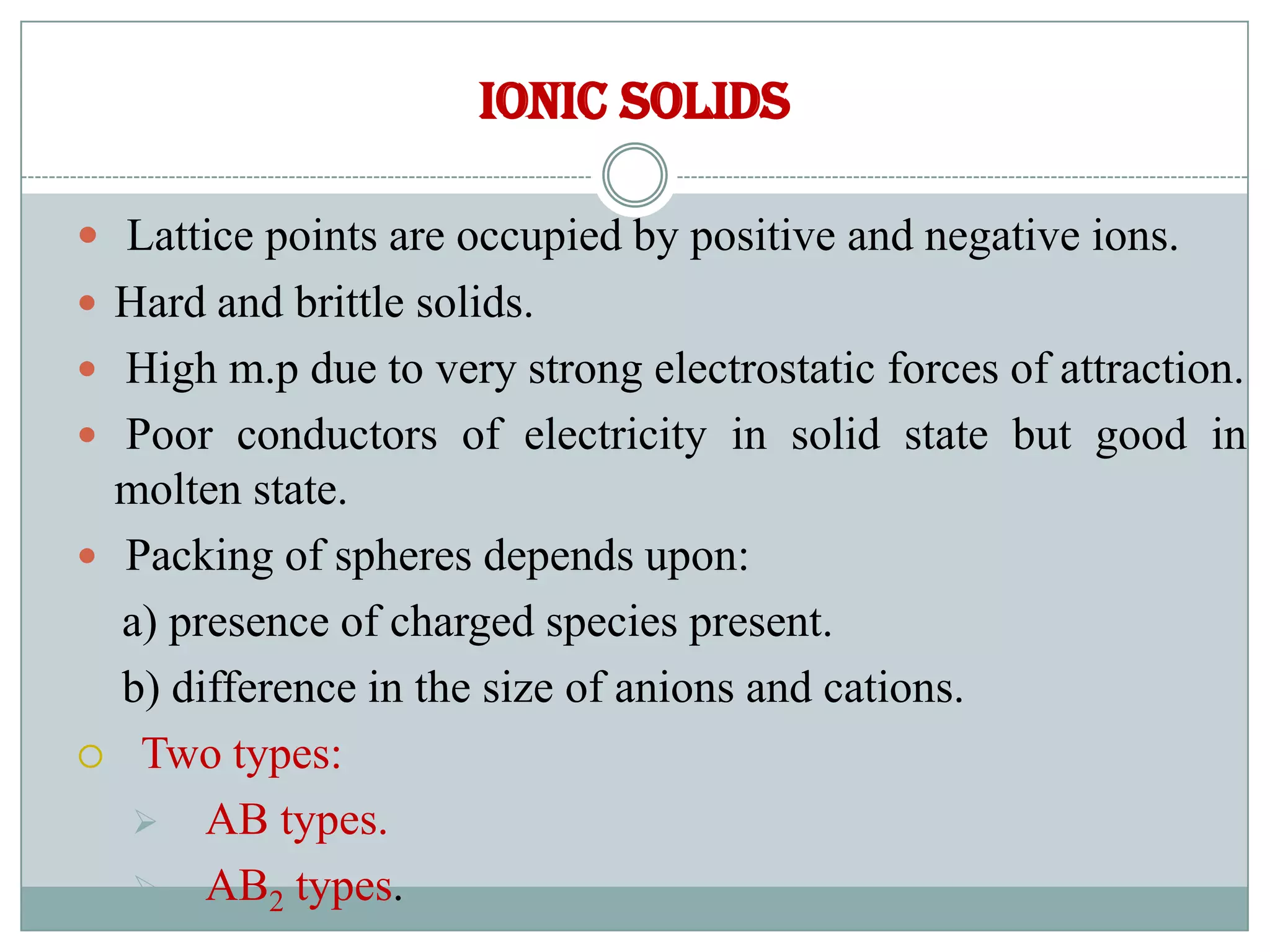
So, what’s holding them back? It’s all about the charges. In ionic solids, you have these things called ions. They’re atoms that have either gained or lost electrons, so they have a definite positive or negative charge. Think of them like tiny magnets, but with electricity's drama.
These oppositely charged ions are attracted to each other. It's a bit like a never-ending game of "tag, you're it!" but way more permanent. They cling together with a strong grip, a force called the ionic bond. It’s a super strong hug that’s hard to break.
This strong ionic bond means the electrons are pretty much glued to their respective ions. They’re not free to wander off and join the electrical parade. They’re too busy being part of the ionic solid’s super-stable structure. It's like they’ve signed a lifelong contract with their ion.
Let’s use an analogy, because who doesn't love analogies? Imagine a packed dance floor at a wedding. Everyone is tightly packed, holding hands with their partners. They're all having a great time, but they're not exactly milling about freely. That's our ionic solid.

Now, if you wanted people to move around the dance floor to deliver a message, it would be pretty tough, right? They're all linked up. This is what happens with electrons in ionic solids. They’re too interconnected, too firmly held in place by their ionic partners.
So, when you try to push electricity through an ionic solid in its solid form, it’s like trying to get someone to skip through that packed wedding dance floor. They just can't. The electrons are tethered. They can’t get loose and carry that electrical charge.
This is why things like table salt, which is sodium chloride (NaCl), are fantastic insulators. You can have a big crystal of salt sitting there, and it’s not going to spontaneously conduct electricity. It’s perfectly happy just being salt. It’s not looking for any electrical drama.
It’s not that they can't conduct electricity under any circumstances. That would be too simple. Ionic solids are just really picky about their conditions. They’re like those people who only drink coffee with exactly two sugars and oat milk.
The magic happens when you mess with the ionic solid a bit. For instance, if you melt an ionic solid, things get interesting. When you melt it, you’re basically giving those ions a bit more wiggle room. They're still charged, and they're still attracted to each other, but they can now slide past each other.

In a molten ionic solid, the ions are free to move. They’re like those wedding guests who finally get off the dance floor to grab some more cake. They're still in the general vicinity, but they're not locked in a fixed position. This movement allows them to carry electrical charge.
So, molten ionic solids can actually be pretty good conductors! It’s like they’ve been liberated from their rigid structure and are ready to participate. It's a whole different ball game when they're all heated up and can move around.
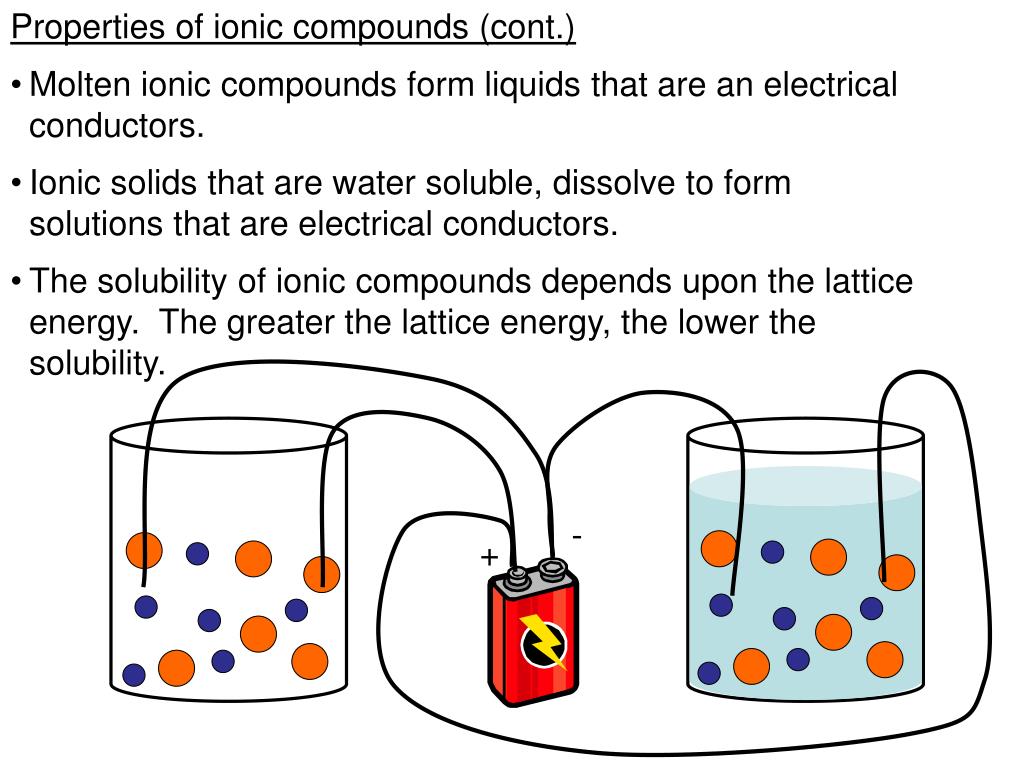
Another way to get them to conduct is to dissolve them in water. When you put an ionic solid in water, the water molecules get in between the ions. They surround the ions and pull them apart. It’s like a referee stepping in to break up a tight hug.
Once the ions are separated and surrounded by water molecules, they are called hydrated ions. These hydrated ions are now mobile. They can move freely through the water. This is why salty water is a conductor of electricity, and pure water is not.
Think of the water as a fantastic facilitator. It’s like a party planner that manages to get everyone on the dance floor and mingling. The ions are still charged, but now they have the freedom to zip around in the watery environment.
So, in their solid, unbothered state, ionic solids are the ultimate chillers. They’re the champions of not conducting electricity. Their tightly bound ions and committed electrons just aren't interested in the electrical hustle and bustle.
They’re like the quiet, respectable citizens of the material world. They do their job, they look neat and tidy, and they don’t cause any electrical trouble. And frankly, there’s something admirable about that. Not everyone needs to be a flashy conductor!
It’s a bit like having a really sturdy, reliable house. It doesn’t do any fancy tricks, but it keeps you safe and sound. Ionic solids are the sturdy houses of the electrical world. They’re built to last, and that includes their resistance to stray electrical currents.
So, next time you encounter a piece of solid salt or any other ionic compound, give it a nod of respect. It’s a master of stillness, a stoic in the face of electrical temptation. It’s probably off doing something more important, like seasoning your food, instead of getting all buzzed up with electricity.
They're not bad conductors; they're just selective conductors. And sometimes, a little bit of selective laziness is exactly what you need to keep things stable and predictable. Let's hear it for the unconductive, stable, and wonderfully ionic solids! They're the unsung heroes of staying put.
It's a bit like a good, strong glue. It holds things together so well that nothing can easily pull them apart. That's the power of those ionic bonds. They're not just bonds; they're like super-glued friendships for atoms.
And when those "friendships" are that strong, the electrons are just part of the package deal. They're not going to bail and go on an electrical adventure when they have such a stable life. It's a commitment, a dedication to the ionic cause.
So, while we might think of electricity as something zippy and fast, ionic solids remind us that sometimes, the best way to be is to be still. To be firmly rooted. To be the unmoving, unconducting marvel that they are.
They’re the introverts of the electrical world, content in their own solid, charged company. And you know what? That's perfectly fine. We all have our strengths, and for ionic solids, their superpower is their steadfast refusal to conduct electricity when they’re in their solid, magnificent form.
It’s a simple concept, really. No free-roaming charged particles? No electricity party. And in ionic solids, those charged particles are too busy holding hands in a very, very strong ionic embrace to go anywhere. They’re committed, and that’s why they’re poor conductors. And that’s okay!
