Why Are Chromium And Copper Exceptions To Electron Configuration
Hey there, ever find yourself staring at something shiny and wondering what makes it so, well, shiny? Or maybe you’ve heard about how important certain elements are for, say, your health or for making things work. Today, we're going to peek under the hood of a couple of these fascinating elements, specifically chromium and copper, and uncover a little secret they’ve been keeping about their electron configurations. Don’t worry, it’s not complicated math; it’s more like a quirky personality trait that makes them stand out from the crowd!
Think of electrons like little kids in a classroom. They have their designated seats, and they’re supposed to fill them up in a specific order, like lining up for recess. We call this the Aufbau principle, which basically means "building up" in German. It’s a neat and tidy system where electrons fill the lowest energy levels first, just like kids filling the front row seats in class before moving to the back.
Now, most elements are perfectly happy to play by these rules. They fill their electron shells and subshells neatly, like a well-behaved class. But then you have our mischievous friends, chromium and copper. They’re like those kids who decide to swap seats or take an extra snack from the communal cookie jar when no one’s looking. They’ve got a little exception to the rule, and it’s actually pretty neat!
The "Almost Full" Feeling
To understand their little quirk, we need to talk about something called subshells. Imagine the classroom again. Within the rows (which are like electron shells), there are smaller groups of seats, called subshells. The ones we’re interested in are called d subshells. These d subshells are super happy when they are either completely empty, completely full, or exactly half full. It's like having a favorite toy – you’re happiest when you have the whole set, or maybe just half of it if you’re sharing nicely, but a random number just feels… incomplete.
So, when chromium and copper are building their electron "classrooms," they get really, really close to having a full or half-full d subshell. It’s like they’re standing at the door of the last seat, looking at it, and thinking, "You know what? I’d be much happier if that last seat was taken (for a full subshell) or if exactly half the seats were taken (for a half-full subshell)."

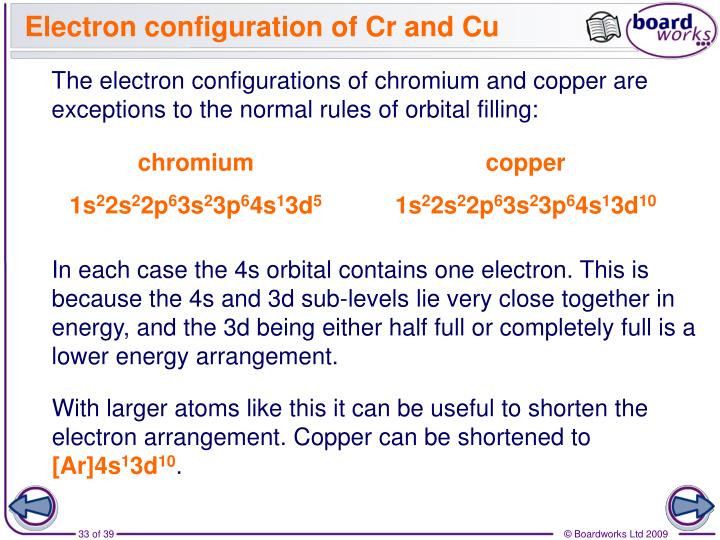
Chromium's Clever Swap
Let’s take chromium, element number 24. If it followed the rules perfectly, its last electrons would be arranged in a way that leaves the d subshell with only four electrons. That’s like having four out of ten seats filled. It’s close, but not quite the happy half-full state. So, chromium, being a bit of a clever clogs, does something sneaky. It takes one electron from the shell before the d subshell (the 4s shell) and moves it into the d subshell.
Voila! Suddenly, the d subshell has five electrons – exactly half full! This makes chromium significantly more stable and happier. Think of it like this: you're packing your lunchbox, and you have your sandwich and your apple. You're almost done, but you have one empty spot. You could just leave it, but you look in your fridge and see a cookie. You realize that putting the cookie in that spot makes your lunchbox feel perfectly balanced, even though it means moving your apple slightly to make room. Chromium feels that same sense of satisfaction when it achieves that half-full d subshell.
Copper's Golden Rule
Copper, element number 29, is much the same. If it played by the standard rules, its d subshell would end up with nine electrons. That's one shy of being completely full. And you know how much elements love to be full! So, just like chromium, copper performs a little electron shuffle. It borrows an electron from the shell in front of it (again, the 4s shell) and tucks it into the d subshell.

Boom! Now, the d subshell in copper is completely full, with ten electrons. This full d subshell makes copper incredibly stable and content. It’s like you’re almost done decorating for a party. You've hung almost all the streamers, but there's just one bare spot on the wall. You look at your box of balloons, and you realize that if you use just one more balloon, the whole room looks perfectly festive. Copper feels that same "perfectly festive" vibe when its d subshell is filled.
Why Should We Care About This Electron Shenanigans?
Okay, so these elements are a little quirky with their electrons. Why should you, an everyday person, care about this? Well, these exceptions are not just random blips; they actually explain a lot of the cool properties these elements have!

Chromium, with its stable electron configuration, is a fantastic element for making things strong and resistant to rust. That’s why you see chromium plating on car parts, faucets, and even some kitchen utensils. It’s tough, it’s shiny, and it doesn’t corrode easily, all thanks to that happy, half-full d subshell. It’s like having a super-powered protective coating!
And copper? Oh, copper is everywhere! Its excellent ability to conduct electricity is a direct result of its electron setup. Think about all the electrical wires in your home, your phone, your computer – many of them are made of copper. Its electrons are happy to flow freely, making electricity travel with ease. It's like having a superhighway for electrons. This is also why copper is used in plumbing and cookware – it’s durable and conducts heat well.
So, the next time you see a shiny chrome bumper, a sturdy copper pot, or even just your phone charging, remember that a little bit of electron rearranging is at play! It’s a reminder that sometimes, breaking the "rules" can lead to something truly special and incredibly useful. These elements are a testament to the fact that even in the seemingly rigid world of atoms, there's room for a little bit of personality and a whole lot of innovation.
