Which Valences Have The Greatest Tendency To Form Ions
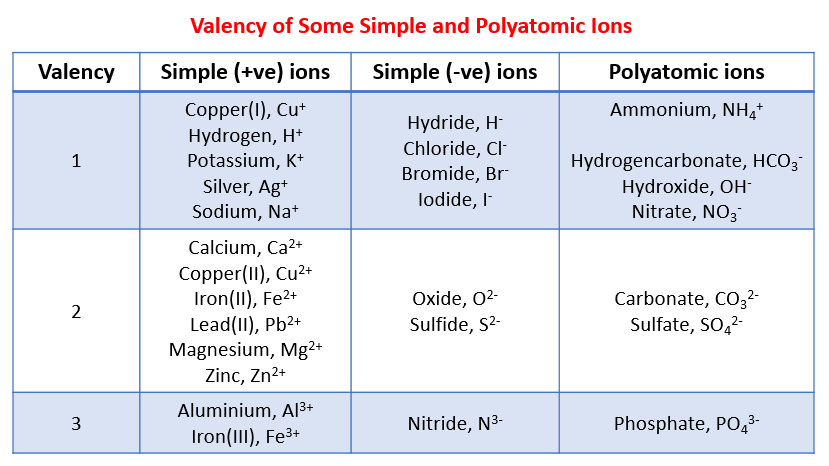
Hey there, science curious folks! Ever wonder what makes the world around you tick? It's not just magic, though sometimes it feels like it, right? Today, we're diving into something super cool that explains a whole lot: valences! Think of valences as the outer shell of an atom, like its little personality trait that dictates how it plays with others. And guess what? Some of these personalities are way more eager to join a party than others. We're talking about the valences that have the greatest tendency to form ions!
Now, "ions" might sound a bit intimidating, but stick with me! It's just a fancy word for an atom that's gained or lost an electron, giving it a little electrical charge. Like a social butterfly at a dance, some atoms are just itching to swap partners (electrons, that is!) to feel more stable and happy. It’s all about achieving that perfect, balanced feeling. And isn't that something we can all relate to? We all want to feel a little more… settled, right?
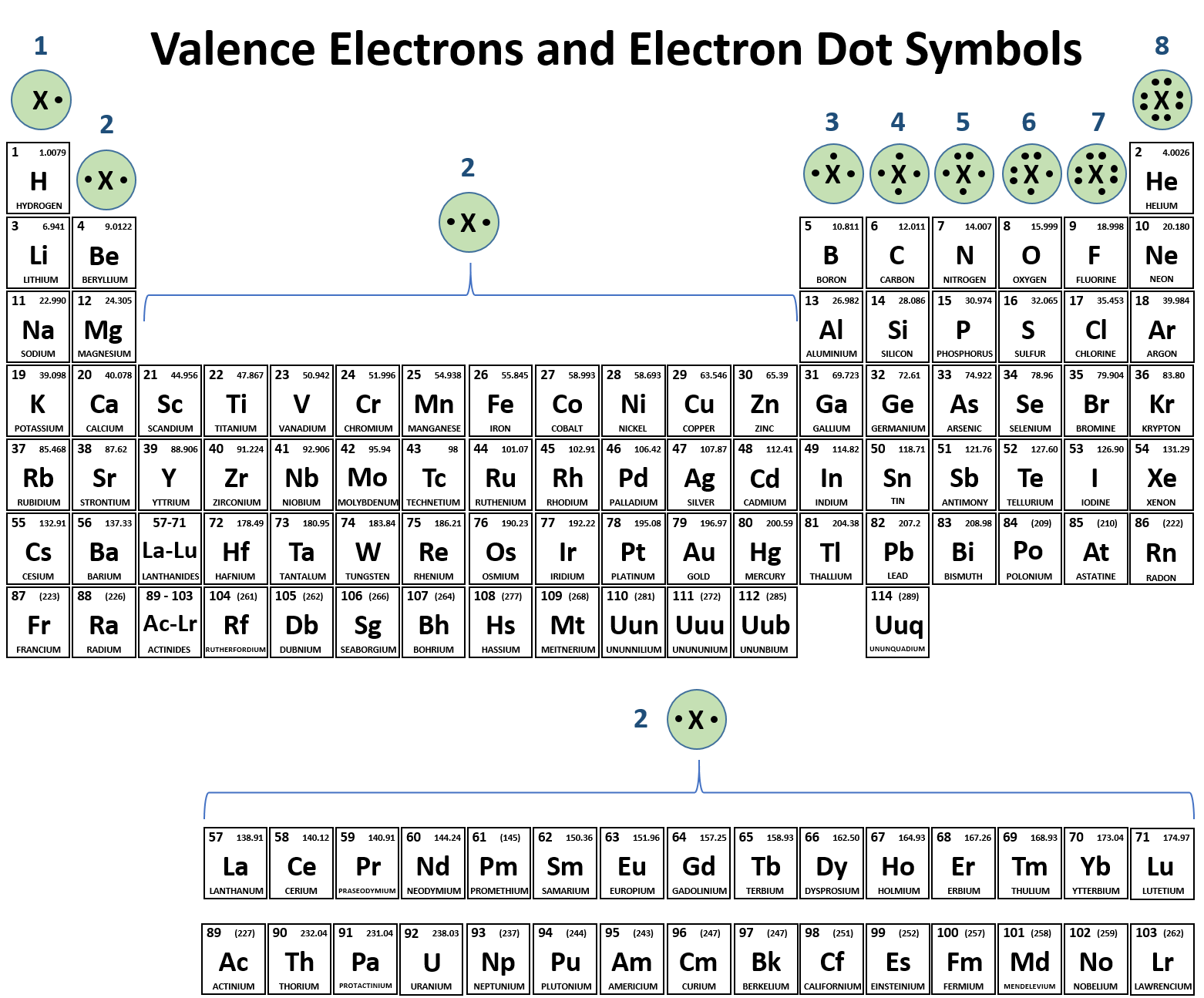
So, which of these eager atoms are the champions of ion formation? Drumroll, please… it’s all about the alkali metals and the halogens! These guys are the rockstars of the ionic bonding world. Seriously, if atoms had a social media presence, these would be the ones with the most followers and the most dramatic updates!
The Super-Eager-To-Lose Crew: Alkali Metals!
Let’s start with the alkali metals. Think Lithium (Li), Sodium (Na), Potassium (K) – you get the picture. These elements are in Group 1 of the periodic table, and they are just bursting with energy. They have one lone electron chilling in their outermost shell.
Imagine you're holding a single, brightly colored balloon at a party. You’ve got it, but it feels a little… incomplete. Wouldn't you be tempted to give it away to someone who’d really appreciate it, maybe someone who has a collection of balloons? That’s kind of how these alkali metals feel!
That single electron in their outer shell is a bit of a nuisance. It’s not enough to make them feel truly "complete" in the way atoms like to feel. So, what do they do? They very happily get rid of it. Poof! Gone!

When they lose that one electron, they become positively charged ions. They’re like, "Yes! I did it! I achieved stability!" They’re so good at this that they don't hesitate. It’s their jam. It’s their superpower, really.
Think about Sodium (Na). You know, the stuff in your table salt? It's desperate to lose that one electron. It's practically begging for someone to take it. And that's how it bonds with Chlorine, which we'll get to in a sec. This simple act of giving away an electron is what makes ionic compounds form, and honestly, it’s the foundation of so much of chemistry!
The Super-Eager-To-Gain Crew: Halogens!
Now, on the flip side, we have the halogens. These are elements like Fluorine (F), Chlorine (Cl), Bromine (Br), and Iodine (I). They’re in Group 17 of the periodic table, and they have almost a full outer shell. They’re just one electron short of that blissful, stable state.
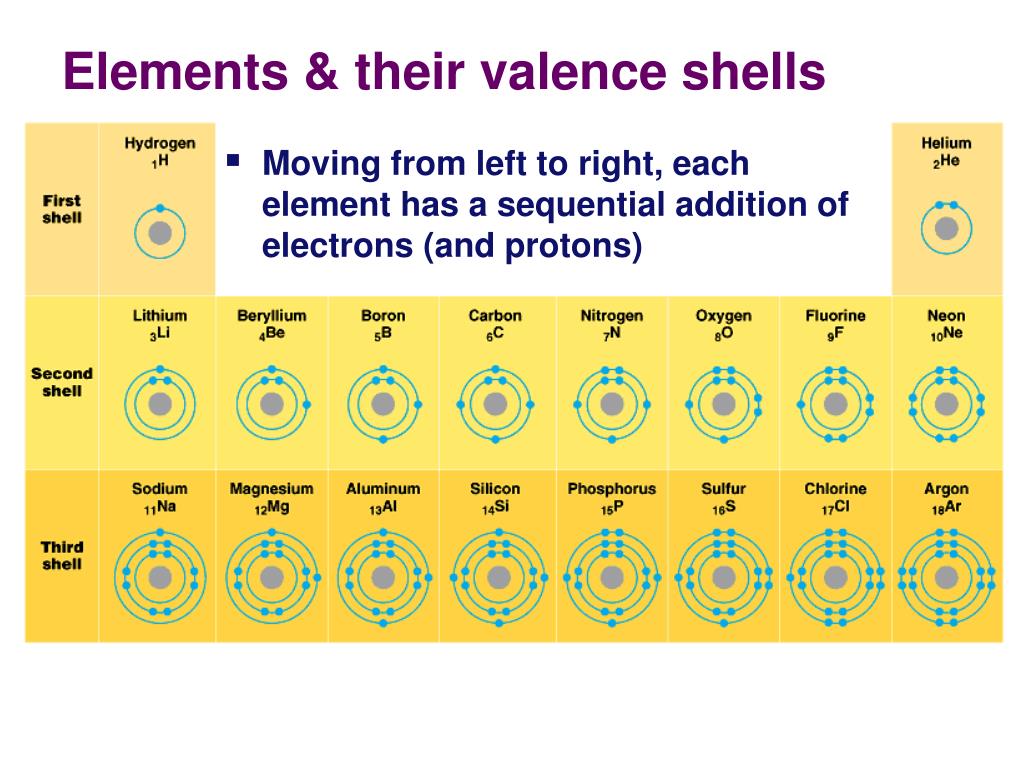
Picture yourself at that same party, but this time, you’re looking at all the amazing snacks. You’re craving just one more bite to feel totally satisfied. That’s the halogens! They are starving for an electron.
When an alkali metal is looking to ditch its extra electron, a halogen is right there, hands (or rather, electron shells) outstretched, saying, "Yes, please! I’ll take it!" It's a perfect match made in the atomic universe. They are so, so good at grabbing that one extra electron.
When they snatch up that electron, they become negatively charged ions. They’re like, "Ahhh, bliss! Finally, I feel complete!" They’re incredibly electronegative, which is just a fancy way of saying they have a really strong pull for electrons. It’s their ultimate goal.
Chlorine (Cl), for instance, is another element found in table salt. It’s practically a magnet for that electron that Sodium is so eager to shed. This give-and-take, this mutual desire for completion, is what makes ionic bonds so strong and so common. It’s like a beautiful, energetic dance.
Why This Ionic Tango is So Fun
So, why is this all so exciting? Because it explains so much about our world! The formation of ions is behind how batteries work, how our bodies send signals, and even how some of the foods we eat are made! It’s not just abstract science; it’s the building blocks of everyday life.
Think about it: the salty snacks you enjoy? That’s sodium and chloride ions doing their ionic thing. The colorful fireworks that light up the sky? Often involve elements that readily form ions. It’s a constant, invisible ballet of atoms seeking stability, and we get to witness its beautiful results all around us.

Understanding these tendencies – the eagerness of alkali metals to give and the hunger of halogens to take – is like having a secret decoder ring for the universe. It’s not about memorizing complex formulas; it's about understanding the motivations, the personalities, of these tiny atomic beings.
It makes chemistry less about tedious rules and more about understanding compelling stories of attraction and completion. It's a narrative of atoms trying to find their perfect balance, and in doing so, creating the very fabric of our reality. Isn't that a wonderfully inspiring thought?
The next time you see something that relies on a chemical reaction, a spark, or even just the salt on your fries, remember the valiant efforts of those valences! The alkali metals and halogens, in their tireless pursuit of ionic bliss, are the unsung heroes of our world. So go forth, embrace this knowledge, and let it inspire you to explore even more of the incredible science that surrounds us. The universe is waiting to be discovered, one ion at a time!
