Which Substance Can Be Classified As An Arrhenius Acid

Okay, confession time. I’ve got a bit of an… unpopular opinion when it comes to acids. Not the scary, lab-coat-wearing kind. More like the everyday, "oh, that’s so acidic" kind. You know, the stuff that makes your taste buds do a little happy dance or, on occasion, a little panicked jiggle.
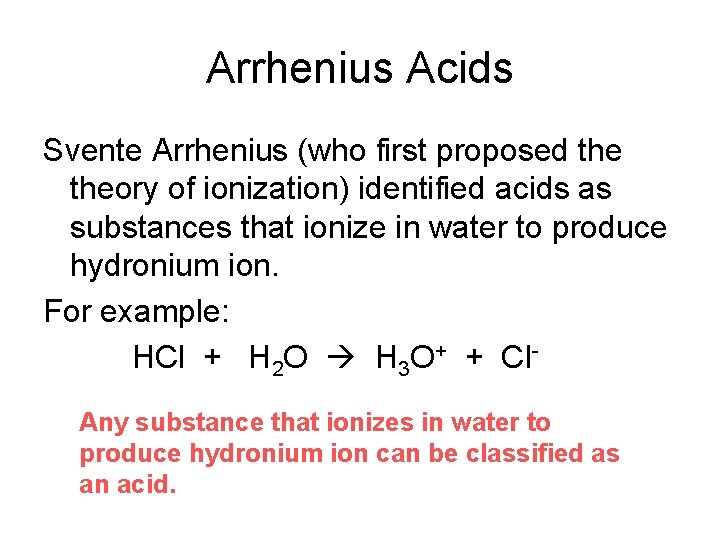
We’re talking about Arrhenius acids, of course. For the uninitiated (and don’t worry, no judgment here!), these are the substances that, when you plop them into water, decide to be generous. They offer up a proton. A hydrogen ion, to be precise. It’s like they have a little H+ to give away to anyone who’s willing to listen. Or, you know, just to the water molecules hanging around.
Now, you might be thinking, "Isn't that… chemistry class?" And yes, yes it is. But let’s be honest, even chemistry class can have its heroes. And in the world of water-loving proton-donors, there are some real rockstars.
The Usual Suspects (You Saw Them Coming)
So, who are these well-behaved proton-givers? Well, the textbook definition usually points to a few familiar faces. Think of the strong acids. They’re the ones who go all-in, donating their protons without hesitation. No second thoughts. They’re like the over-enthusiastic friend at a party, ready to share everything.
Hydrochloric acid (HCl) is probably the most famous. You’ve heard of it, right? It’s in your stomach, helping you digest that giant pizza. So, technically, your stomach is a mini acid factory. Pretty cool, or slightly alarming? You decide.
Then there’s sulfuric acid (H₂SO₄). This one is a bit more… intense. It’s used in car batteries and in making fertilizers. It’s not something you want to be casually sipping. Unless you’re a superhero, perhaps. A very, very chemically resistant superhero.

And let’s not forget nitric acid (HNO₃). It’s used in making explosives. So, yeah, definitely not for your morning coffee. Unless you’re aiming for a very memorable wake-up call.
These guys are the classic examples. They readily release that H+ in water. It’s their whole deal. They’re the A-listers of the Arrhenius acid world, strutting their stuff in every lab manual ever written.
The Tangy Tourists (Taste Buds Rejoice!)
But my opinion? It gets a little more… fun. Because what about the things we eat and drink that also have that acidic zing? The ones that make you pucker up in the best way possible? Are they not also, in their own delicious way, Arrhenius acids?
Let’s consider citric acid. It’s practically the mascot for the citrus fruit industry. Oranges, lemons, limes – they’re all packed with it. When you squeeze a lemon into your water, you’re essentially introducing a little bit of a proton-donating party into your glass. The citric acid molecules are just there, chilling, and when they hit the water, poof! A little H+ is liberated. It’s the sour taste that makes your mouth water, literally.
And don’t even get me started on vinegar. That stuff is pure acetic acid (CH₃COOH), with a dash of water. When you’re making salad dressing, or pickling cucumbers, you are absolutely engaging with an Arrhenius acid. The acetic acid molecules are just waiting for their chance to release a proton. It’s a delightful, tangy release.
Think about it: when something tastes sour, it’s often because of the presence of these acids. Our tongues are practically designed to detect these proton-donating wonders. So, why do we reserve the "Arrhenius acid" title for the industrial powerhouses when our favorite foods are doing the same thing, just in a tastier package?
The "Unpopular Opinion" Section (Hold Onto Your Hats!)
Here’s where my theory gets a little… wild. I believe that anything that gives you that distinct sour sensation, and is known to release H+ ions in water (even if it's in small amounts), should get a nod in the Arrhenius acid hall of fame. It's about function, right? If it donates a proton and makes things taste sour, let's celebrate it!

So, in my humble, and perhaps slightly heretical, opinion, lactic acid found in yogurt and sauerkraut? Arrhenius acid. Malic acid in apples? Arrhenius acid. Even the stuff that makes your soda fizz with that characteristic tang? Yep, likely some carbonic acid (H₂CO₃) playing the proton-giving game.
It just makes sense to me. It’s like saying a celebrity is only a "star" if they win an Oscar, ignoring all the incredible actors who deliver amazing performances but haven’t had that particular piece of hardware. These food acids are performing their chemical duty, donating those protons with gusto.
They might not be as dramatic as sulfuric acid in a battery, but they’re certainly more pleasant to encounter on a daily basis. They add zest to our lives, quite literally. They make food interesting. They turn bland into… well, not so bland.
Why the Snobbery, Science?
I just don’t understand the gatekeeping. Arrhenius acids are defined by their ability to increase the concentration of hydrogen ions in an aqueous solution. That’s it. That’s the whole shebang. If a substance does that, and it's safe enough for us to consume and enjoy, why wouldn't we classify it as such?

It’s like having a club with a very exclusive guest list. Meanwhile, all these perfectly lovely, proton-donating individuals are standing outside, wondering why they can’t get in. They’re just trying to share their H+!
Perhaps it’s the strength. The strong acids are really good at it. They’re like the Olympic athletes of proton donation. But that doesn’t mean the amateur joggers, the ones with a more mellow approach, aren’t participating in the same sport.
So, the next time you’re enjoying a slice of grapefruit, or a sip of kombucha, or even just a tangy pickle, take a moment. Appreciate the chemistry. Appreciate the Arrhenius acid that’s making it all happen. They might not have the same spotlight as their more volatile cousins, but they’re just as deserving of the title, in my book. They’re the unsung heroes of our kitchens, and honestly, our taste buds are all the better for it.
And who knows, maybe one day, the official definition will expand to include all the delicious proton-donors out there. Until then, I'll be over here, enjoying my tangy, sour, and undeniably acidic world, with a knowing wink to my favorite Arrhenius acids. Cheers to H+!
