Which Statements Describing Chemical And Nuclear Reactions Are True

So, I was at my nephew Leo’s birthday party last week. He’s turning ten, and you know how it is with ten-year-olds – they’re fascinated by anything that goes boom or involves a bit of a chemical explosion. His dad, bless his adventurous heart, decided to set up a “science experiment” for the party. It involved baking soda, vinegar, and a whole lot of red food coloring. The idea was to create a volcano that erupted with “lava.”
It was a riot, honestly. The moment that fizzy mixture hit the vinegar, this magnificent (and slightly terrifying) pink foam gushed out of the plastic bottle volcano, splattering the delighted (and slightly sticky) kids. Leo was over the moon. He kept shouting, “It’s a chemical reaction! Just like in the cartoons!” And for a moment, watching all that fizz and pop, it really did feel like pure magic. But then, as the last of the foam subsided and we were all wiping down surfaces, I got to thinking. Leo was right, of course. It was a chemical reaction. But what exactly is a chemical reaction? And more importantly, how is it different from what happens deep inside the sun, or in a nuclear power plant? Are they just… bigger booms?
This got me down a bit of a rabbit hole, as things tend to do with me. I started wondering about the fundamental differences between the everyday magic of a baking soda volcano and the immense power of nuclear reactions. It turns out, they’re not just different in scale; they’re fundamentally different beasts. And understanding those differences can be pretty eye-opening. So, grab yourself a cuppa, and let’s dive into the wonderful world of chemical versus nuclear reactions. It’s not as scary as it sounds, I promise!
The Everyday Dance of Atoms: Chemical Reactions
Let’s start with what we saw at Leo’s party – chemical reactions. Think of it as atoms throwing a party, but they’re careful not to break up their own families. In a chemical reaction, the outer electrons of atoms are the main players. These are the little guys on the fringes, the ones that are happy to mingle and form new friendships (bonds).
When baking soda (sodium bicarbonate) meets vinegar (acetic acid), the molecules get a little shake-up. The sodium bicarbonate breaks apart, and the acetic acid does too. They then rearrange themselves, forming new molecules like sodium acetate, water, and carbon dioxide gas. That carbon dioxide gas is the real star of the show in our volcano experiment. It needs space, and it needs it now, so it bursts out, creating all that delightful foam and fizz. See? It’s just a molecular dance, a bit of shuffling and reforming.
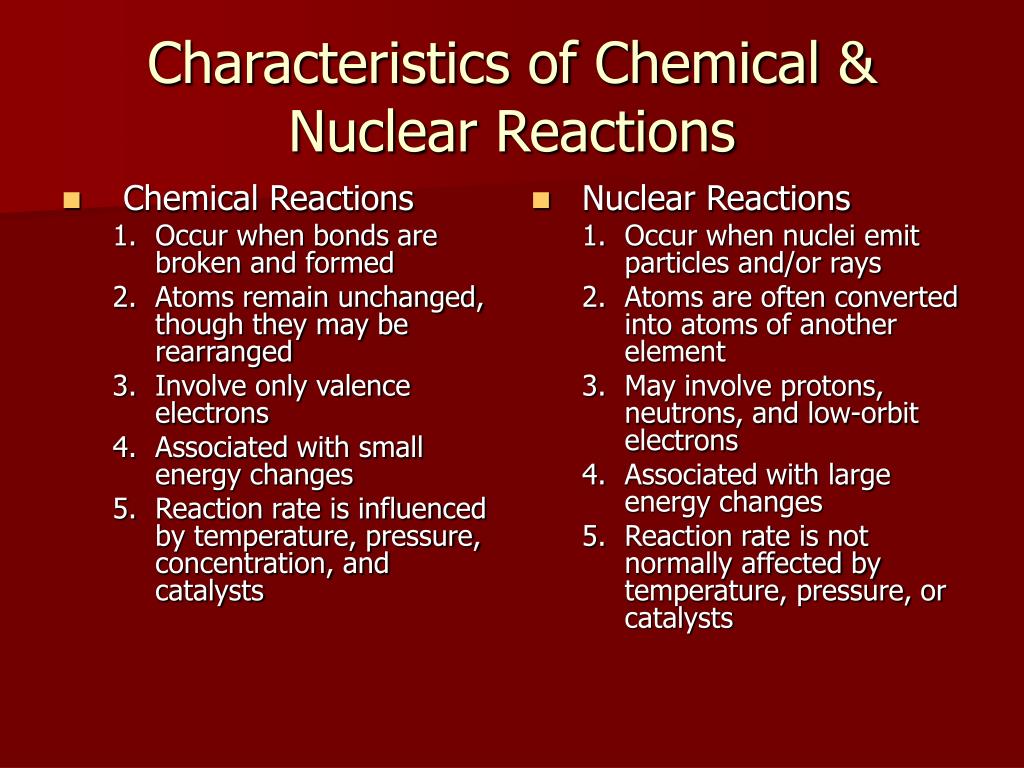
The key here is that the nuclei of the atoms remain unchanged. The protons and neutrons chilling in the center of each atom? They’re totally undisturbed. It’s like the party guests are rearranging the furniture, but the house itself (the nucleus) stays exactly as it was. We’re talking about the formation of new substances with new properties. Water and salt are made, but the atoms that made them up are still the same fundamental building blocks.
This is why chemical reactions are so prevalent in our daily lives. Burning wood, rusting iron, digesting food, photosynthesis in plants – all of these are chemical reactions. They involve breaking and forming chemical bonds, and the amount of energy released or absorbed is generally much smaller compared to nuclear reactions. It’s all about the electron shells, folks.
So, when you’re baking a cake, or your phone’s battery is powering up, or even when your body is converting that delicious pizza into energy, you’re witnessing the magic of chemical reactions. It’s a constant, dynamic process that underpins so much of our existence. Pretty neat, right?
The Big Guns: Nuclear Reactions
Now, let’s talk about the “nuclear” part of things. This is where we crank up the volume, and I mean way up. Nuclear reactions are not about those flashy electron friends on the outside; they’re about what’s happening deep, deep inside the atom – in the nucleus itself. This is where the protons and neutrons hang out, and let me tell you, they’re a much more tightly packed and energetic bunch.
In nuclear reactions, the nuclei of atoms are transformed. This can happen in a couple of main ways: nuclear fission and nuclear fusion. You’ve probably heard of these terms, especially in relation to nuclear power plants or, you know, the sun.
Nuclear fission is like taking a big, unstable nucleus and carefully cracking it into smaller pieces. Imagine a really overstuffed piñata. When you hit it just right, it breaks open, releasing all the candy (energy!) and smaller bits (new nuclei and particles). In fission, a heavy nucleus, like uranium, absorbs a neutron. This makes it unstable, and it splits into two or more smaller nuclei, releasing a tremendous amount of energy, along with more neutrons. These new neutrons can then go on to cause more fission events, creating a chain reaction. This is the principle behind nuclear reactors and atomic bombs.
Then there’s nuclear fusion. This is the opposite: taking small nuclei and forcing them together to form a larger nucleus. Think of it as the cosmic equivalent of a super-strong hug. This process requires immense heat and pressure, which is why it’s what powers stars like our sun. When hydrogen nuclei fuse together to form helium, they release an absolutely staggering amount of energy. It’s the ultimate energy source, in a way.
The key takeaway here is that in nuclear reactions, the elements themselves can change. Fission of uranium produces different elements. Fusion of hydrogen produces helium. This is a far cry from chemical reactions where the atoms, while rearranging their electron partnerships, remain the same elements. The identities of the atoms are being fundamentally altered.
Untangling the Truths: Which Statements Hold Up?
Alright, so we’ve got our two main categories. Now, let’s tackle some statements and see if they’re true or false, keeping our new knowledge in mind. This is where it gets fun, like a little quiz for your brain!
Statement 1: "In a chemical reaction, the nucleus of an atom is always altered."
Hmm, what did we say about chemical reactions? They’re all about the electrons, right? The nucleus is left untouched. So, this statement is… false. The nucleus is the unmoving core, while the electrons are doing the cha-cha. Remember the baking soda volcano? The carbon atoms are still carbon atoms, the oxygen atoms are still oxygen atoms. Their electron arrangements just changed to make new molecules.
+true+of+nuclear+reactions.jpg)
Statement 2: "Nuclear reactions can change one element into another."
Now, this is where we talked about fission and fusion. When a nucleus splits or merges, the number of protons changes. And what determines the element? That’s right, the number of protons! So, if the number of protons changes, the element changes. This is precisely what happens in nuclear reactions. Therefore, this statement is… true. Think of those sci-fi movies where they transmute elements – while often dramatized, the underlying principle of changing elements is rooted in nuclear processes.
Statement 3: "Chemical reactions involve the sharing or transfer of electrons between atoms."
Going back to our electron party analogy. Chemical bonds, whether they’re covalent (sharing) or ionic (transferring), are formed by the interaction of valence electrons. This is how molecules are built. So, this statement is… true. This is the fundamental mechanism of chemical change. It’s all about those eager outer electrons finding new partners.
Statement 4: "Nuclear reactions release significantly less energy than chemical reactions."
Okay, let’s think about the energy scale here. A typical chemical reaction, like burning a log, releases a good amount of heat. But a nuclear reaction? The energy released from fission or fusion is millions of times greater than from a comparable chemical reaction. That’s why a tiny bit of nuclear fuel can power a city for a long time, or why a small nuclear weapon is so devastating. So, this statement is… false. It’s the other way around; nuclear reactions release vastly more energy.
Statement 5: "The conservation of mass is always strictly observed in chemical reactions, but can be violated in nuclear reactions."
This is a bit of a nuanced one. In chemical reactions, mass is indeed conserved. The total mass of the reactants equals the total mass of the products. All the atoms are just rearranged. However, in nuclear reactions, we enter the realm of Einstein’s famous equation, E=mc². This tells us that mass and energy are interchangeable. During nuclear reactions, a tiny amount of mass is converted into a huge amount of energy. So, while the total mass-energy is conserved, the apparent mass of the particles might decrease slightly as it becomes energy. Therefore, saying mass is strictly observed in nuclear reactions is misleading. This statement is… true, but with a crucial understanding of mass-energy equivalence in nuclear processes. The strict conservation of mass alone is a hallmark of chemical reactions, not nuclear ones.

Statement 6: "Nuclear fission and nuclear fusion are types of chemical reactions."
Hold on a second. Did we just spend all that time explaining how nuclear reactions affect the nucleus and change elements, while chemical reactions only involve electrons and rearrange molecules? If fission and fusion alter the nucleus and change elements, they can’t possibly be chemical reactions. So, this statement is… false. They are distinct processes, with vastly different scales of energy and fundamental interactions.
Statement 7: "In both chemical and nuclear reactions, new substances are always formed."
Let’s think about this. In a chemical reaction, you start with reactants (like baking soda and vinegar) and end up with products (like sodium acetate, water, and carbon dioxide). These are definitely new substances with different properties. So, for chemical reactions, true. Now, what about nuclear reactions? Fission produces different elements, which are new substances. Fusion produces helium from hydrogen, also a new substance. So, it seems like this is true for both. Therefore, this statement is… true. You’re either making new molecules or new elements.
Statement 8: "Radioactivity is a characteristic of chemical reactions but not nuclear reactions."
Radioactivity, that spontaneous emission of radiation, is a result of unstable atomic nuclei. Which type of reaction involves the transformation of nuclei and often leads to unstable isotopes? Nuclear reactions! Chemical reactions, on the other hand, deal with the electron shells and don't inherently make nuclei unstable in the way radioactive decay does. So, this statement is… false. Radioactivity is fundamentally linked to nuclear processes, not chemical ones.
The Takeaway: Not All Explosions Are Created Equal
So, there you have it! It’s pretty fascinating how the universe plays by such different rules depending on whether we’re looking at the outer electrons or the inner nucleus. Leo’s volcano was a brilliant display of chemical change, a beautiful rearrangement of atoms that’s safe and common. But the power of the sun, or a nuclear reactor, or even a star going supernova – that’s a whole other ballgame, governed by the immense forces within the atomic nucleus.
Understanding these differences isn’t just for science buffs. It helps us appreciate the world around us, from the food we eat to the energy that powers our lives. It also reminds us that while things that go boom can be exciting, the underlying mechanisms can be vastly different and have very different consequences. So next time you see something fizz, pop, or even explode (responsibly, of course!), take a moment to consider: is it an electron rearrangement or a nuclear showdown? Your answer might just surprise you!
