Which Statement Is True About Electron Shielding Of Nuclear Charge
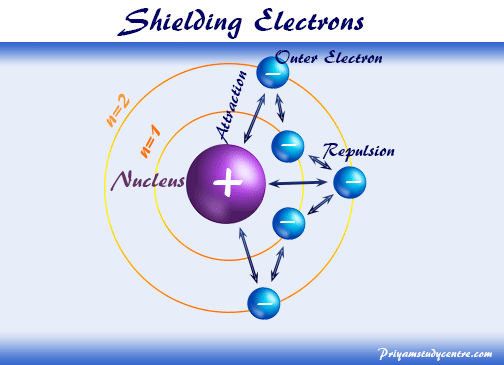
Hey there, science enthusiasts and curious cats! Ever wondered what's really going on inside those tiny, bustling worlds we call atoms? It's like a mini-universe, full of energetic characters and their own special rules.
Today, we're going to dive into something super cool: electron shielding. Think of it as the atom's own VIP section, where some electrons get a little bit of a break from the intense attention of the positively charged nucleus. It’s a fascinating dance, and understanding it is like unlocking a secret level in the game of chemistry!
So, how does this whole shielding thing work? Imagine the nucleus at the center of an atom is like a super popular celebrity. Everyone wants its attention, and it's got this amazing, powerful pull.
But then, you have these electrons zipping around. Some of them are super close to the nucleus, practically in its autograph line. Others are further out, maybe chilling in the nosebleed seats, enjoying the show from a distance.
Now, here’s where the fun begins. The electrons closer to the nucleus act like a bunch of enthusiastic fans, blocking some of the nucleus's powerful charisma from reaching the electrons further away. It’s like they’re holding up little signs that say, "Hey, the main star is over here, don't worry about it too much!"
This "blocking" effect is what we call electron shielding. The inner electrons essentially "shield" the outer electrons from the full, unadulterated force of the positive nucleus. They're like a protective layer, a cozy blanket of sorts.
So, if you were an electron way out in the suburbs of the atom, you'd feel a bit less of that central pull, right? That's the magic of shielding! It changes how strongly those outer electrons are held.
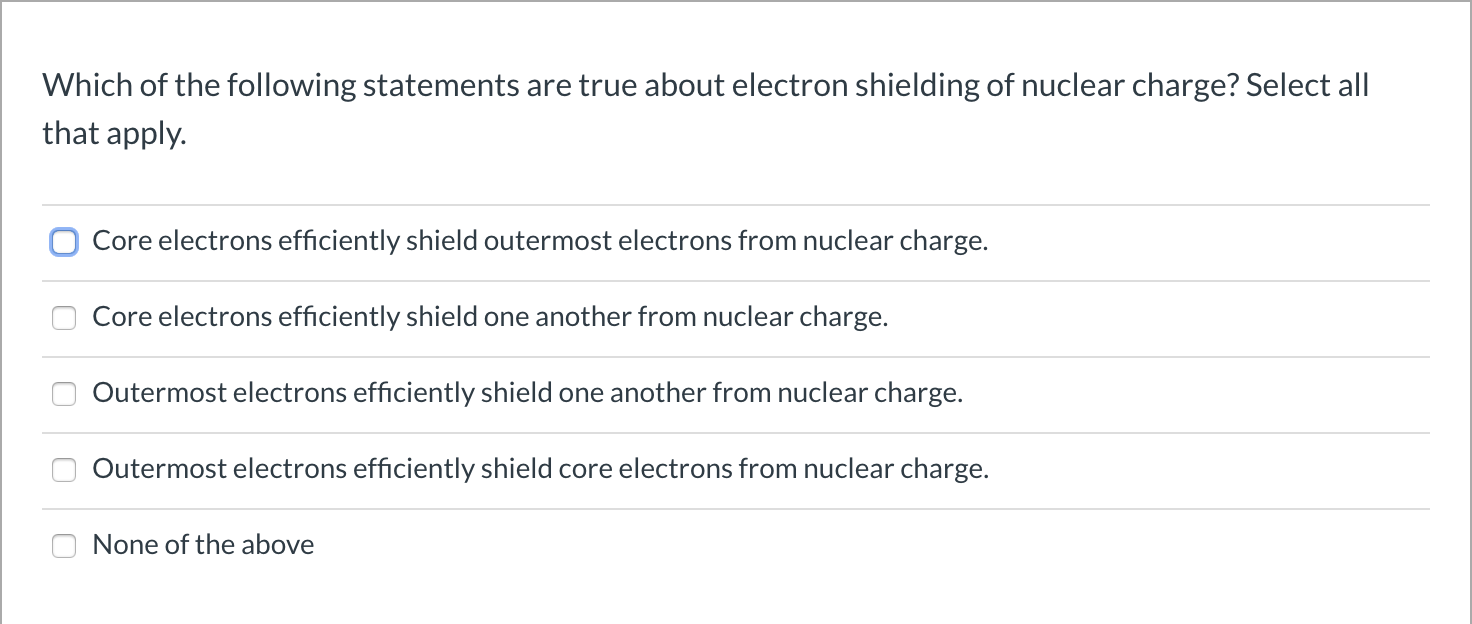
Now, let's get to the juicy part: which statement truly captures the essence of this atomic phenomenon? It's like a riddle, and the answer is hiding in plain sight, if you know where to look!
The Truth Unveiled!
Get ready for a revelation that’ll make your atomic-understanding heart sing! When we talk about electron shielding, there’s one statement that really nails it. It’s the one that gets the vibe just right.
Think about it: the nucleus is positively charged, right? It’s like a giant magnet, attracting all those negatively charged electrons. But the electrons aren't just floating around in a void; they're organized in shells, like different floors in a building.
The electrons in the very inner shells are the ones doing the most important shielding work. They're like the bouncers at the club, keeping the inner circle tight and intercepting some of that magnetic pull. These inner electrons, being closer to the nucleus, have a stronger attraction to it.

Because they're so close and so keen on the nucleus, they sort of absorb or deflect a significant portion of the nucleus's positive charge. This means the electrons in the outer shells don't feel the full brunt of the nucleus's positive pull. They feel a "reduced" or "shielded" nuclear charge.
So, the key takeaway is this: inner electrons shield outer electrons from the full nuclear charge. It’s a simple concept with profound implications for how atoms behave!
Imagine the nucleus as a super-bright spotlight. The electrons in the first shell are like tiny mirrors directly in front of the spotlight. They bounce some of that light away.
The electrons in the second shell, further out, don't get as much direct light because of those first-shell mirrors. They're still getting some light, but it's definitely dimmer than if there were no mirrors at all. This is electron shielding in action!
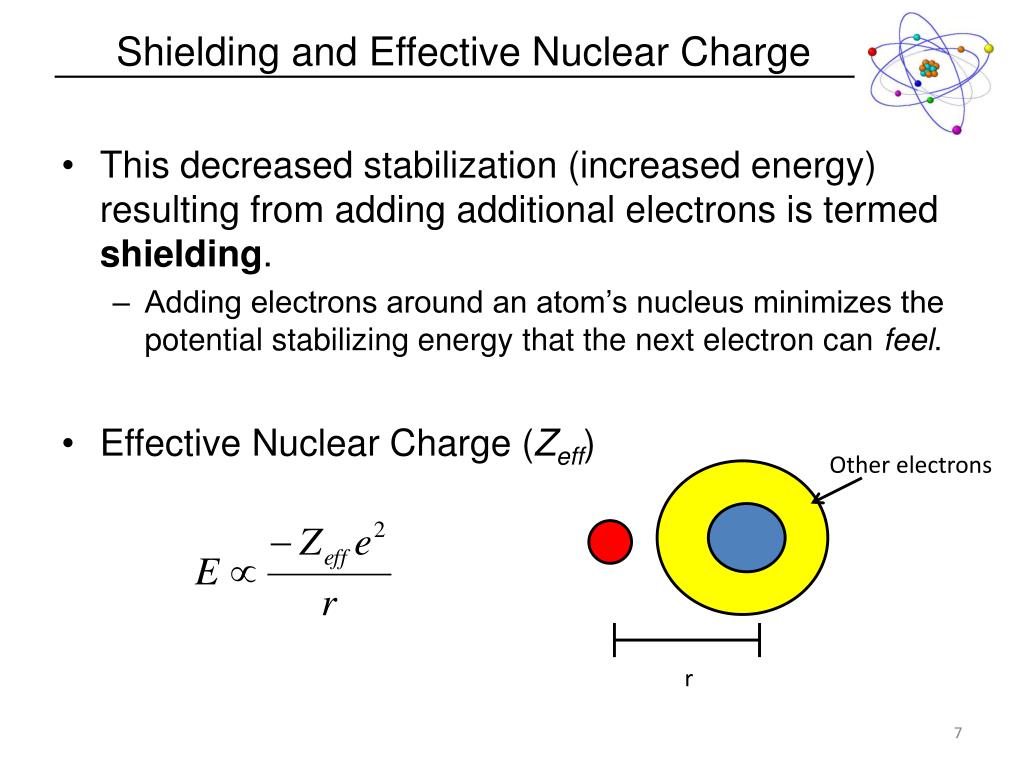
This shielding effect is super important for understanding things like atomic size and how atoms interact with each other. It's the unsung hero of chemical bonding!
Why It Matters (Spoiler: A Lot!)
So, why should you care about these invisible electron shields? Well, it’s like knowing the secret handshake to a club that determines how much an atom wants to grab onto its electrons.
If the outer electrons feel a weaker pull from the nucleus, they're more likely to wander off or join up with another atom. This is the foundation of chemical reactions! It’s all about who’s holding on tightest and who’s looking for a new connection.
Think of it like this: if the nucleus is the ultimate prize, the shielding effect determines how easy it is for other atoms to "steal" or "share" those prize outer electrons. A stronger nuclear pull means the atom is a bit more possessive, a bit less likely to share.
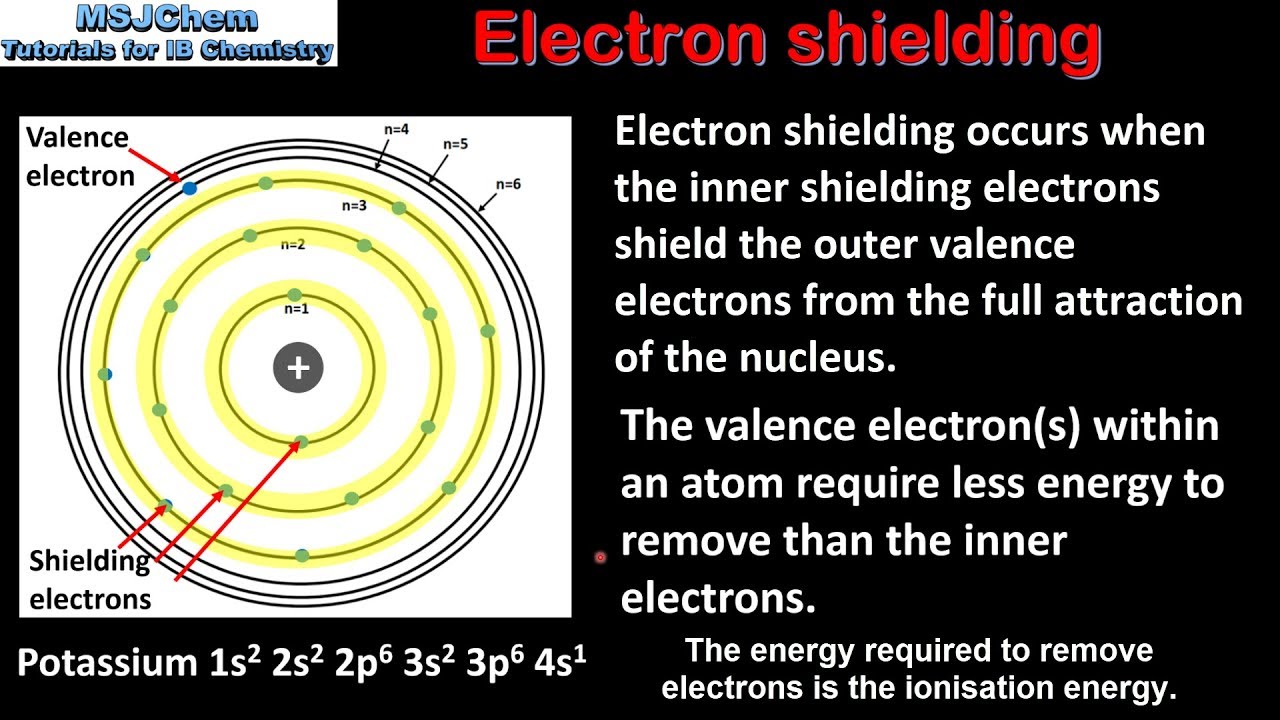
The concept of electron shielding is a fundamental principle in chemistry. It helps us predict how elements will behave and why they form the compounds they do. It’s like having a cheat sheet for the entire periodic table!
It's the reason why certain elements are super reactive, always eager to make new friends, while others are a bit more reserved, content in their own atomic company. The subtle dance of attraction and repulsion, all governed by these invisible shields!
So, the next time you look at an atom, remember the inner electrons are like the loyal bodyguards, protecting the outer electrons from the full, intense gaze of the nucleus. They’re the unsung heroes, making sure everything stays balanced in the atomic world.
It’s a beautiful, intricate system, and understanding even a little bit of it can make you feel like a microscopic marvel. You're now one step closer to understanding the building blocks of everything! Isn't that just electrifying?
Remember: It's the inner electrons that do the heavy lifting when it comes to shielding the outer electrons from the nucleus's powerful positive embrace.
So there you have it! The truth about electron shielding is wonderfully straightforward and incredibly important. Keep exploring, keep wondering, and keep enjoying the amazing world of science!
