Which Statement Is True About A Proton And An Electron
Imagine the tiniest dancers in the universe, constantly twirling and spinning. These aren't your average ballerinas, but the fundamental building blocks of everything we see and touch: protons and electrons. They’re like the cosmic equivalent of peanut butter and jelly, or perhaps a grumpy old man and a cheerful puppy, always interacting in fascinating ways.
Now, let’s play a little game of "Which Statement Is True About A Proton And An Electron?" Think of it as a cosmic scavenger hunt for the most important, mind-boggling, and sometimes downright silly facts about these tiny powerhouses. We’re going to explore their quirks and characteristics, and by the end, you’ll be seeing the world a little differently.
The Sizeable Difference
First off, let's talk about size. If a proton were a basketball, an electron would be so incredibly tiny, it would be like trying to find a single speck of dust on that basketball. Seriously, it’s that minuscule! This difference in size is one of the most striking things about them.
It’s almost as if the universe decided, "Let's make one thing really solid and substantial, and then just sprinkle a few of these nearly invisible things around for good measure!" It's a bit like having a giant teddy bear and then a bunch of tiny fairy wings attached to it.
The Charge Game: Opposites Attract!
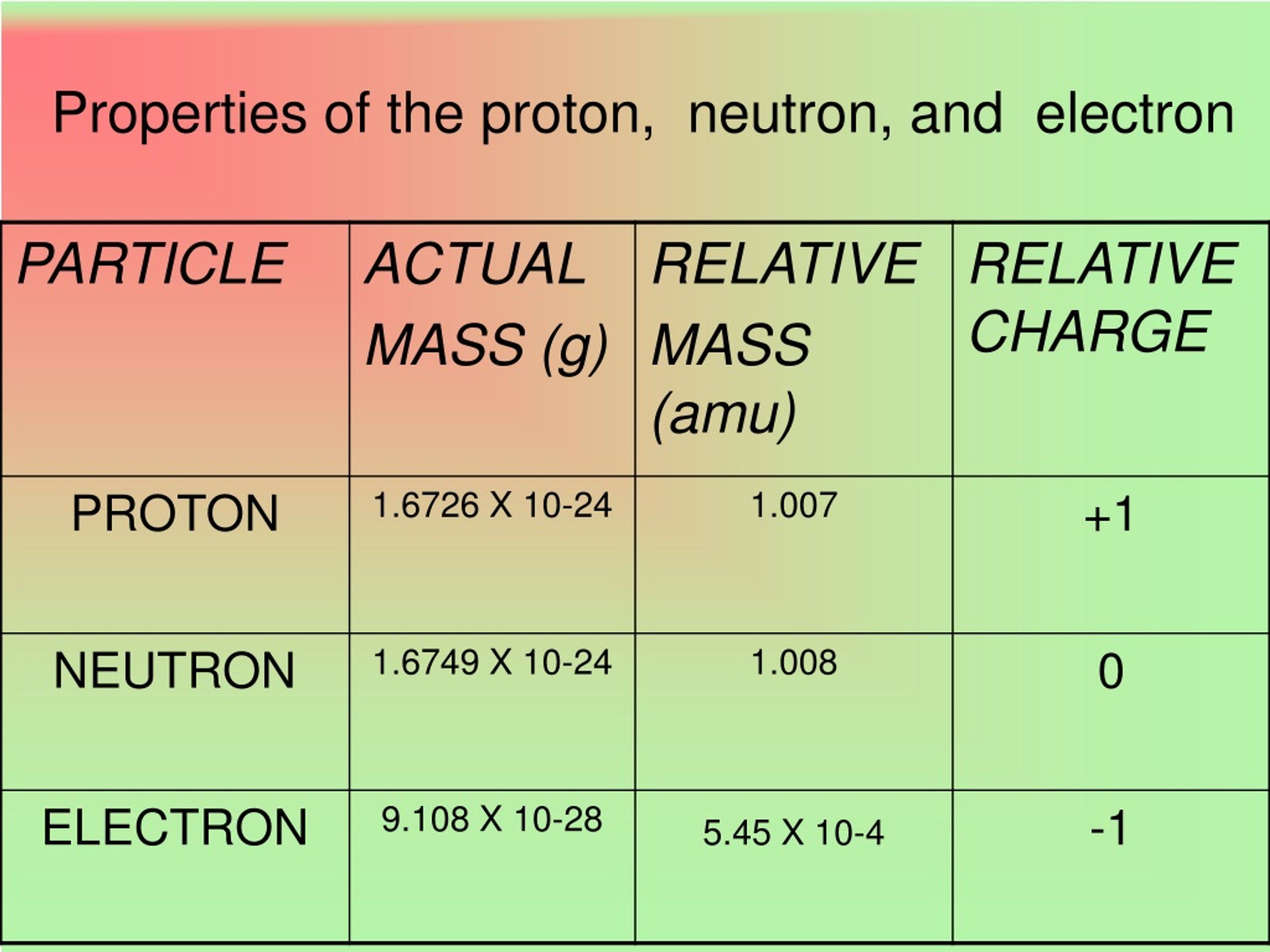
Here’s where things get really interesting, and a little bit like a romantic comedy. Protons have a positive charge, like a ray of sunshine. Electrons, on the other hand, have a negative charge, sort of like a tiny, electric storm cloud.
And what happens when you put opposites together? They attract! It’s the universal law of dating, but on a subatomic level. This attraction is what holds atoms together, creating the stable structures that make up your favorite chair, your morning coffee, and even your own amazing self.
It’s this constant push and pull, this magnetic embrace, that keeps the universe from flying apart. Imagine if gravity worked like this – your socks would always stick to your pants! But thankfully, it’s just the charges of these little guys.
Where Do They Live?
Protons are typically found hanging out in the very center of an atom, in a cozy little neighborhood called the nucleus. They’re like the grumpy, yet essential, mayors of atom town, holding down the fort. They’re quite stable and don’t like to wander off.
Electrons, however, are the rebels of the atomic world. They zip and zoom around the nucleus in designated energy levels, like hyperactive teenagers on a sugar rush. They’re much more mobile and can even jump from atom to atom, which is pretty cool when you think about it.

This movement of electrons is actually how electricity works! So, next time you flip a light switch, remember it's all thanks to these speedy little troublemakers. They're the unsung heroes of our plugged-in lives.
The Weighty Matter
When we talk about weight in the atomic world, protons are the heavyweight champions. They contribute most of an atom's mass. An electron, in comparison, is practically weightless.
It's like trying to weigh a bowling ball versus a feather. The bowling ball (the proton) is where all the heft is. The feather (the electron) is just along for the ride, making things happen without adding much to the scale.
This difference in mass is crucial for how atoms behave and interact. It’s the foundation for everything from the density of a diamond to the lightness of a cloud. Who knew such tiny things could have such a big impact on weight?

Stability and Change
Protons are remarkably stable. They’re like the sturdy old oak tree in a forest, not prone to sudden changes. They’ve been around since the early days of the universe, and they’re expected to stay that way for a very, very long time.
Electrons, on the other hand, are the social butterflies. They’re constantly in motion, interacting with other atoms and even jumping between them. This dynamism is what allows for chemical reactions and the formation of all the diverse molecules we find in nature.
Think of protons as the reliable anchors, and electrons as the energetic connectors. Together, they create a beautiful dance of stability and change that builds the universe. It’s a perfect partnership, even with their vast differences.

The Astonishing Fact
So, which statement is true about a proton and an electron? It's a question that might seem simple, but it leads us down a rabbit hole of amazing discoveries. They are vastly different in size, with the proton being like a giant and the electron a minuscule speck.
Their charges are like two sides of a coin, one positive and one negative, leading to a powerful attraction that binds atoms together. The proton is the heavy anchor, while the electron is the light, zippy traveler, influencing everything from electricity to chemical bonds.
But here’s the truly heartwarming part: despite their differences, these two fundamental particles are essential to each other. Without protons holding things together, electrons would have nowhere to orbit. Without electrons buzzing around, atoms wouldn't be able to form the complex structures that make life possible. They are, in their own tiny way, perfectly complementary, proving that even the smallest parts of the universe can create something truly magnificent.
"The more I learn about the universe, the more I realize it's just a giant, beautiful, and slightly chaotic dance between these tiny particles."
