Which Statement Correctly Describes Glucose C6h12o6
Hey there, science curious friends! Ever wondered about that sweet stuff, glucose? You know, the sugar that powers you up? Well, there's a really cool way to describe it, and it's like unlocking a tiny, fascinating secret about our everyday lives. It's a bit like finding out your favorite snack has a superhero name!
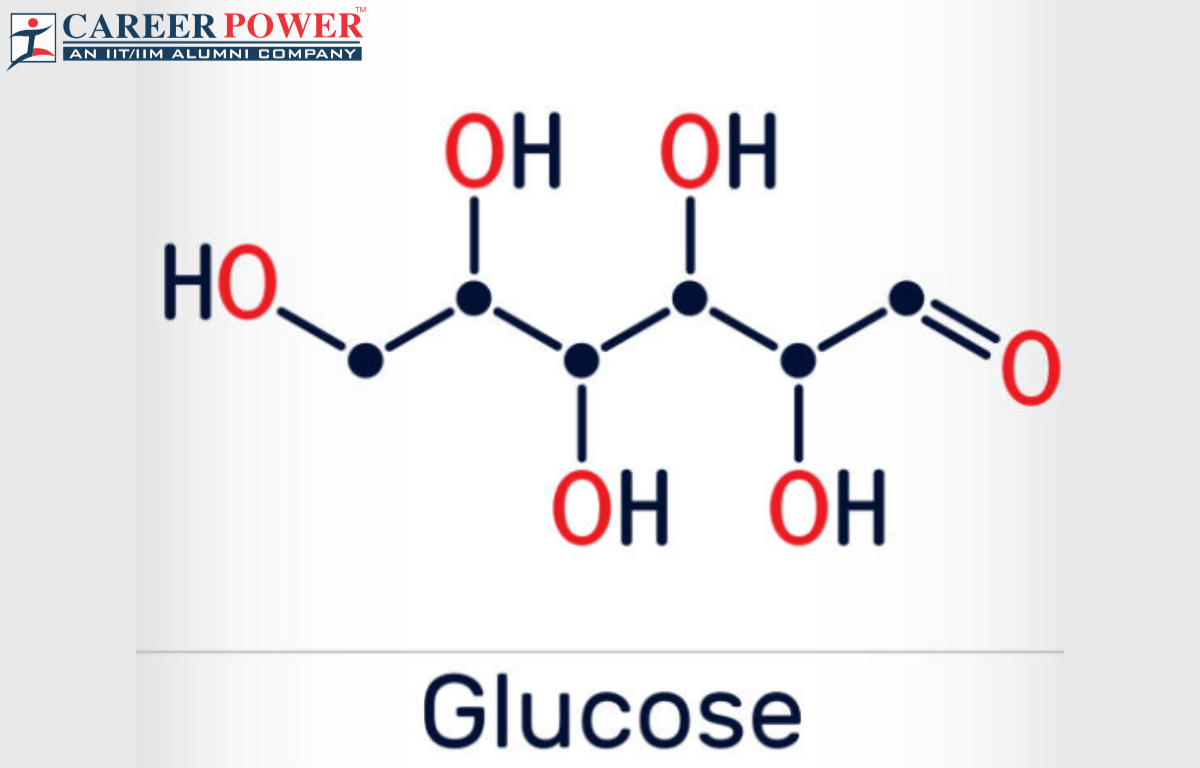
We're talking about glucose, the star of our show today. Its scientific name might sound a bit fancy: C6H12O6. But don't let that scare you! This is where the real fun begins, because understanding this little formula tells us so much about this amazing molecule.
Imagine you're building with LEGOs. The formula C6H12O6 is like the blueprint for our glucose LEGO creation. It tells us exactly what bricks we need and how many of each. Pretty neat, right?
So, what does C6H12O6 actually mean? Let's break it down. The 'C' stands for carbon. Think of carbon as the sturdy, central bricks in our LEGO castle. Glucose has 6 of these carbon bricks.
Then we have the 'H', which stands for hydrogen. Hydrogen is like the smaller, more flexible connecting pieces. In glucose, we have a whopping 12 hydrogen pieces. That's a lot of connectors!
And finally, the 'O' represents oxygen. Oxygen bricks are like the special pieces that help everything stick together and do its job. For glucose, we have 6 oxygen pieces.
So, when you see C6H12O6, it’s just a shorthand way of saying: "This molecule is made of 6 carbon atoms, 12 hydrogen atoms, and 6 oxygen atoms, all linked together in a very specific, special way." It's like a secret code that scientists use to describe things.
Now, why is this so exciting? Because this exact arrangement, this precise number of atoms (6 carbon, 12 hydrogen, 6 oxygen), is what makes glucose... well, glucose! It's the magic combination that gives it all its amazing properties.
This isn't just any random collection of atoms. They are arranged in a ring-like structure, sort of like a hexagonal donut. This shape is super important for how glucose behaves and how our bodies use it.
.jpg)
Think of it this way: if you had 6 carbon, 12 hydrogen, and 6 oxygen atoms, but they were all jumbled up in a messy pile, it wouldn't be glucose. It might be something else entirely! The specific way they bond together is the key.
And that's where the statement that correctly describes glucose really shines. It's not just about listing the ingredients; it's about the recipe and the final delicious outcome!
One of the most entertaining things about glucose is its role as our body's primary fuel. When you eat something with carbohydrates, your body breaks it down into glucose. It then travels through your bloodstream, ready to be used by your cells for energy.
Imagine your body as a super-efficient car. Glucose is the premium gasoline that keeps the engine running smoothly. Without it, you'd be running on empty!
This is why you feel a burst of energy after eating something sweet (though it's best to get your glucose from healthy sources like fruits and whole grains!). It's your body saying, "Yay, fuel!"
So, a statement that correctly describes glucose would highlight this crucial role. It's a simple sugar, a monosaccharide, and a vital energy source.
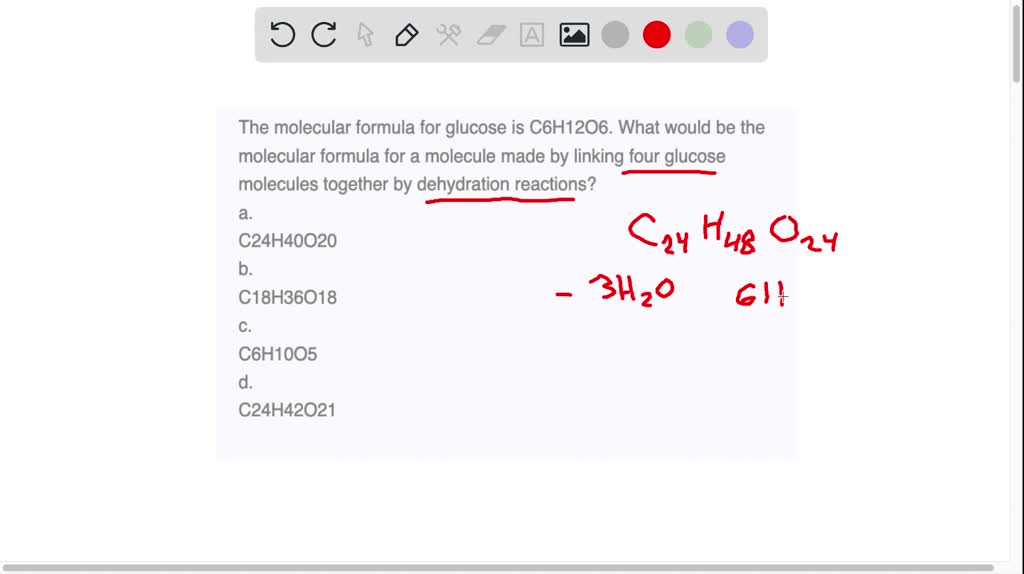
Let's get a little more specific about what makes it so special. The "mono" in monosaccharide means "one." So, it's a single sugar unit, unlike more complex sugars that are made of multiple units linked together.
This simplicity makes glucose easily digestible and ready for your cells to use. It's like a pre-cut slice of cake, ready to be enjoyed without any fuss.
Another fun fact is that glucose is the most abundant monosaccharide found in nature. It’s everywhere! Plants make it through photosynthesis, and then animals eat plants (or eat other animals that ate plants) to get it.
This means that when you're enjoying a juicy apple or a sweet carrot, you're consuming the very same type of sugar molecule that fuels your own body. It's a beautiful natural connection!
So, when you encounter a statement about glucose, look for clues that point to its structure (C6H12O6) and its function as a primary energy provider. These are the hallmarks of a correct description.
Consider this: A statement might say, "Glucose is a molecule with the chemical formula C6H12O6 that serves as the main source of energy for living organisms." This is a pretty solid description, right?
It tells you what it's made of (the formula) and what it does (energy source).
Or, it might emphasize its sweetness and its role in our diet. After all, it's the "sugar" we often talk about!
.jpg)
But the truly entertaining part is realizing how this one little molecule, with its specific arrangement of atoms, is so fundamental to life. It's the invisible engine powering everything from a tiny ant to a giant whale, and yes, even you!
The way glucose is processed in our bodies is a marvel of biology. It involves complex pathways like glycolysis, the Krebs cycle, and oxidative phosphorylation, all working together to extract energy from glucose.
While we don't need to delve into those complex pathways right now, knowing that glucose is the starting point for so much energy production is truly fascinating.
Think about a runner crossing the finish line. That burst of speed? A lot of that comes from glucose being efficiently converted into energy.
So, the correct description will likely mention its status as a monosaccharide and its importance in cellular respiration, the process by which our cells create energy.
It's not just about being sweet; it's about being the essential building block for life's energy needs. It's the universal currency of energy for cells.

The chemical formula C6H12O6 is like the ingredient list on a magical potion. It tells you exactly what’s inside that makes the magic happen.
And the magic it performs is providing the power for your brain to think, your muscles to move, and your heart to beat. Pretty impressive for a simple sugar!
So, when you're looking at different descriptions of glucose, keep an eye out for the ones that capture its essence: its precise atomic composition and its indispensable role in powering life.
It's like finding the best way to describe your favorite superhero. You'd talk about their powers, their origin story, and why they're so important. Glucose has its own amazing origin (photosynthesis!) and incredibly vital powers.
A statement that correctly describes glucose will be both accurate and illuminating. It will help you understand why this molecule is so central to our existence and the natural world around us.
It's a molecule that's both incredibly simple in its formula and profoundly complex in its biological significance. That duality is what makes it so endlessly interesting.
So next time you hear about glucose, remember the LEGO blueprint C6H12O6 and the amazing energy it unlocks. It’s more than just sugar; it’s the fuel of life!
