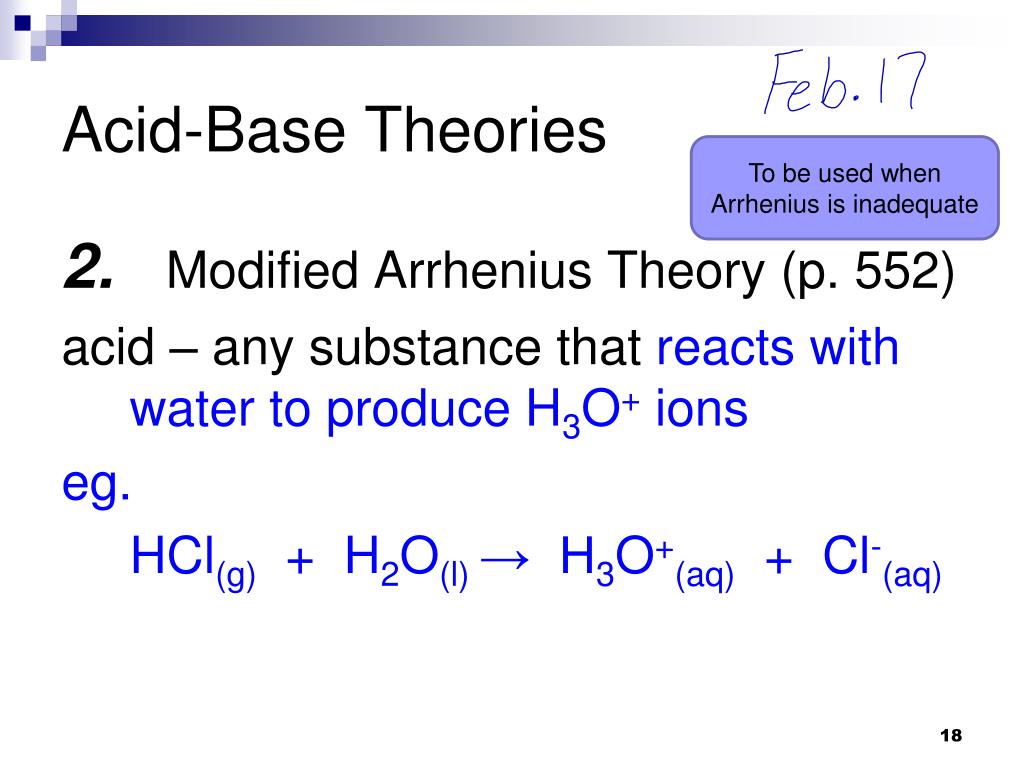
Which Statement Concerning Arrhenius Acid Base Theory Is Not Correct
Ever stumbled upon a particularly vibrant color palette or a delightfully complex flavor profile and thought, "How did they do that?" Well, often, the secret ingredient to that magic isn't some mystical muse, but a surprisingly simple, yet profoundly creative, scientific principle: the Arrhenius acid-base theory! Now, before you picture bubbling beakers and daunting equations, let's reframe this. Think of it less as dry textbook fodder and more as a whimsical framework for understanding how things interact, influencing everything from the tang of your lemon sorbet to the way paint pigments create dazzling hues.
For artists, hobbyists, and the perpetually curious casual learner, the Arrhenius theory, in its broadest sense, offers a fresh perspective. It’s about understanding the fundamental nature of substances – their tendency to donate or accept certain "parts" (in the real theory, it’s ions, but for our creative purposes, think of them as tiny building blocks). This understanding can unlock a world of experimentation. Imagine a painter, delving into the world of natural dyes. Knowing how acidic or alkaline substances interact with fibers can lead to an array of unexpected and beautiful color variations. A baker, wanting to perfect that fluffy muffin, might intuitively grasp how acidic ingredients (like buttermilk) react with alkaline ones (like baking soda) to create lift – a delicious outcome driven by chemical interaction!
The beauty of this concept lies in its adaptability. You don't need a full laboratory. Think about the difference in flavor when you add a squeeze of lime (acidic) to a savory dish versus a sprinkle of baking soda (alkaline) to your pancake batter. These are everyday examples of acid-base reactions at play. Even in gardening, understanding the pH of your soil (a measure of its acidity or alkalinity) can help your plants flourish. It’s about observing these reactions, whether they produce a pleasing tartness, a vibrant hue, or a sturdy structure. Variations abound: the gentle neutralization that softens a harsh flavor, the controlled addition of an acid to cure a food, or even the delightful fizz when a fizzy drink is created by mixing acidic and alkaline components.
Want to try a bit of this creative chemistry at home? It’s simpler than you think! Try a classic science experiment by mixing baking soda and vinegar. Observe the vigorous fizzing – that's a visible representation of an acid-base reaction. Or, experiment with natural indicators: boil red cabbage in water, then divide the liquid into several cups. Add a little lemon juice (acidic) to one and a touch of baking soda (alkaline) dissolved in water to another. Watch the colors transform – a simple yet captivating demonstration of pH! For bakers, actively thinking about the interaction between acidic and alkaline ingredients when following a recipe can lead to a deeper understanding and more successful results. It encourages a thoughtful approach to ingredient selection.
Ultimately, the joy of exploring the Arrhenius acid-base theory, even in its simplified, creative interpretation, comes from the sense of discovery and empowerment it offers. It’s about realizing that the world around us is a constant dance of interactions, and understanding these basic principles can unlock a deeper appreciation for the everyday wonders we often take for granted. It’s a reminder that science, at its heart, is a remarkably creative and inspiring force, capable of adding a little extra sparkle to our lives.
