Which Statement Best Describes The Properties Of Ionic Compounds

Hey there, science explorers! Ever wondered about those super interesting tiny building blocks that make up, well, pretty much everything? Today, we're diving headfirst into the dazzling world of ionic compounds. Forget those stuffy textbooks; we're going to uncover what makes these guys so special, and trust me, it's more exciting than finding a forgotten ten-dollar bill in your old jeans!
So, imagine you've got a bunch of little rebels. Some are positively charged, like tiny superheroes with a mission, and others are negatively charged, like cool, calm characters who just go with the flow. When these opposite characters meet, BAM! They get attracted to each other like magnets. This magnetic attraction is the heart and soul of ionic compounds.
The best way to describe what makes ionic compounds tick is to talk about their amazing properties. Think of them as the rock stars of the chemical world, with a few signature moves that set them apart. Let's break down what makes them so utterly fantastic and, dare I say, a little bit magical!
The "Stick Together Like Super Glue" Property!
First off, ionic compounds are basically the ultimate clingers. Because of that super strong attraction between their charged bits (we call them ions!), they form these really organized, rigid structures. Imagine a perfectly stacked tower of LEGO bricks, but at a microscopic level. It’s like they’ve all decided to hold hands forever and never let go!
This incredible stickiness means they have super high melting and boiling points. You can't just casually melt or boil them with a regular lighter. We're talking about needing some serious heat, like the kind you’d find in a volcano or a blast furnace. So, when you see something like table salt (that's sodium chloride, a classic ionic compound!) sitting happily on your counter, know that it takes a whole lot of energy to get those little ions to loosen up.
Think about it: that's why your cooking salt doesn't just evaporate when you're simmering some pasta. It’s got that ionic backbone, that super-strong bond, keeping it all together. It's the chemical equivalent of saying, "Nah, not today!" to heat.
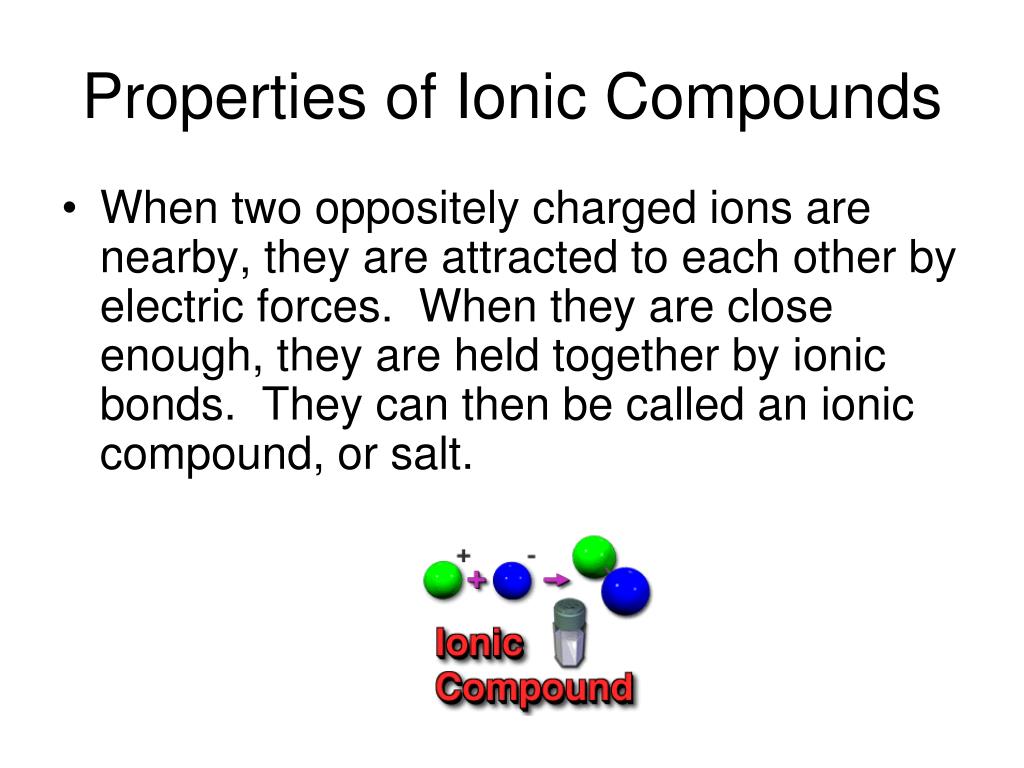
The "Let's Get This Party Started (When Dissolved!)" Property!
Now, here’s where things get really interesting and a bit like a surprise party. While ionic compounds are tough cookies in their solid form, many of them are total social butterflies when you mix them with water. They don't just dissolve; they break apart into their individual charged ions, ready to mingle!
And guess what these charged ions do? They can conduct electricity! It’s like opening up a superhighway for tiny electrical charges to zoom around. This is why solutions of many ionic compounds are amazing conductors. Imagine turning on a light bulb with a salty solution – pretty neat, right?
So, while a solid block of table salt won't do much for your electronics, dissolve it in water, and suddenly you've got a tiny electrical conductor on your hands. It’s this ability to free up those charged particles that allows electricity to flow. It’s a bit like these compounds have a secret switch that turns on their conductivity when they get a good splash of water.

The "Hard as a Diamond, But... Oops!" Property!
Because of those strong attractions, ionic compounds are generally quite hard. They resist scratching and denting, much like precious gems. Trying to scratch a piece of ionic crystal is like trying to tickle a statue – it's pretty darn unyielding!
However, there's a little catch to their toughness. While they’re hard, they can also be quite brittle. Imagine a perfectly crafted glass sculpture. It’s beautiful and hard, but a sharp tap can send it shattering into a million pieces. Ionic compounds are a bit like that.
A good, strong whack can break those organized structures apart. It’s because the impact can shift the layers of ions, bringing like charges next to each other. And you know what happens when positive meets positive, or negative meets negative? They repel each other like a couple of cats who just discovered they both want the same sunny spot! This repulsion causes the whole structure to fracture.
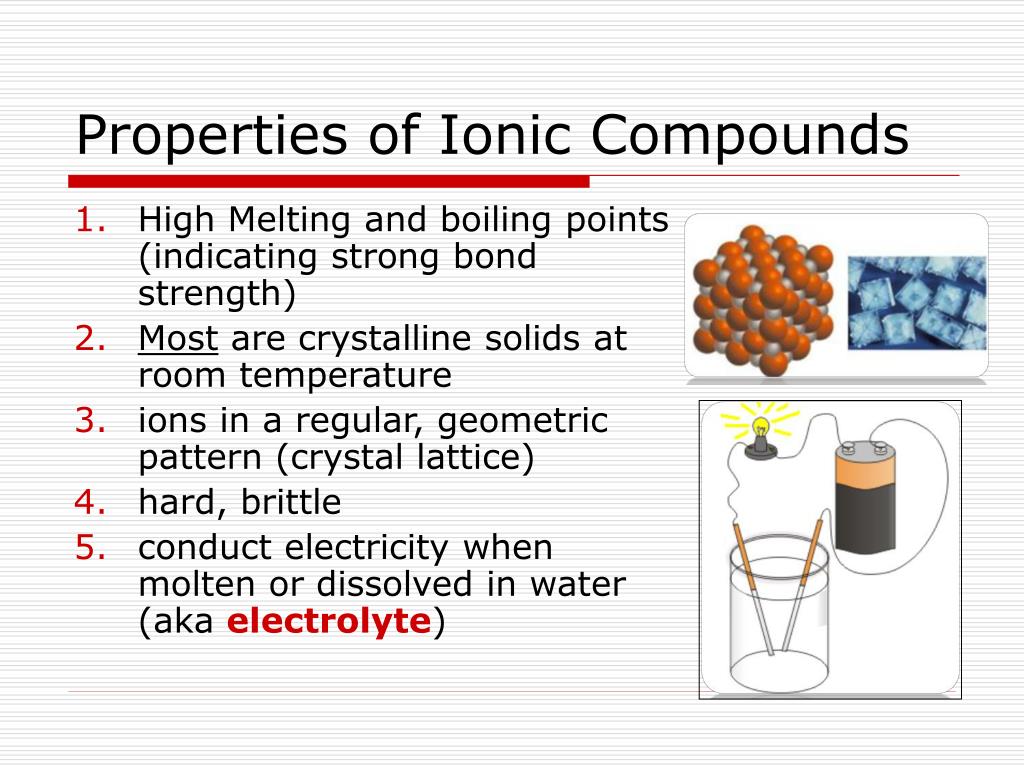
The "When Heated, They Don't Just Smoke and Fizzle" Property!
We touched on this with their high melting points, but it’s worth reiterating. Unlike some other types of compounds that might decompose or turn into a gooey mess when heated, ionic compounds just stubbornly hold their form until they reach their incredibly high melting points. They are the marathon runners of the chemical world, not easily giving up!
They don't typically break down into simpler, funky-smelling gases or turn into something entirely different with moderate heating. They just get hotter and hotter until they finally decide to become a liquid, and then an even hotter gas, if you can even get them there!
This makes them super stable in many high-temperature applications. Think about ceramics or the coatings on your pots and pans – many of these rely on the incredible heat resistance provided by the strong bonds in ionic compounds.
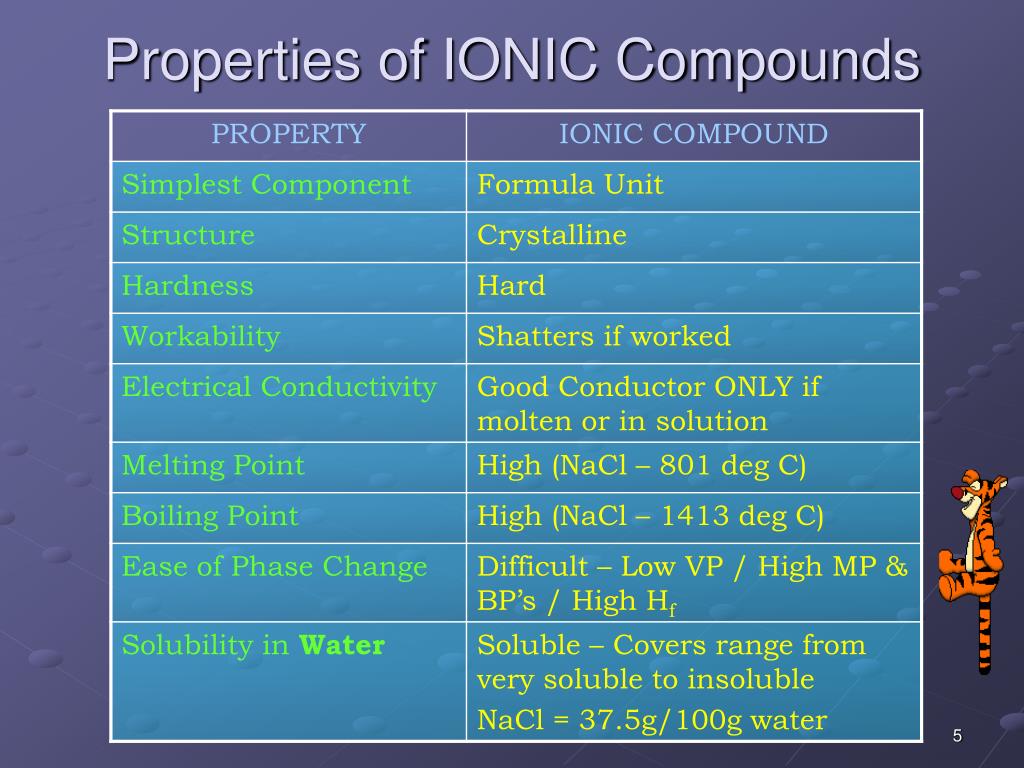
So, Which Statement Reigns Supreme?
Alright, drumroll please! If we had to pick the statement that best captures the essence of these amazing ionic compounds, it would be something that highlights their strong electrostatic attraction between oppositely charged ions, leading to high melting/boiling points, brittleness, and conductivity when dissolved or molten.
It’s that fundamental idea of opposite charges grabbing hold of each other with all their might that dictates their behavior. They are tough, they are conductive (under the right circumstances!), and they require a serious energy investment to change their state. They are the steadfast, sometimes surprising, but always fascinating backbone of so much around us!
So next time you sprinkle some salt on your fries or admire a sparkly gem, give a little nod to the awesome power of ionic compounds. They're the unsung heroes, the chemical workhorses, and a testament to the cool, interconnected nature of the universe. Keep exploring, and keep being amazed!
