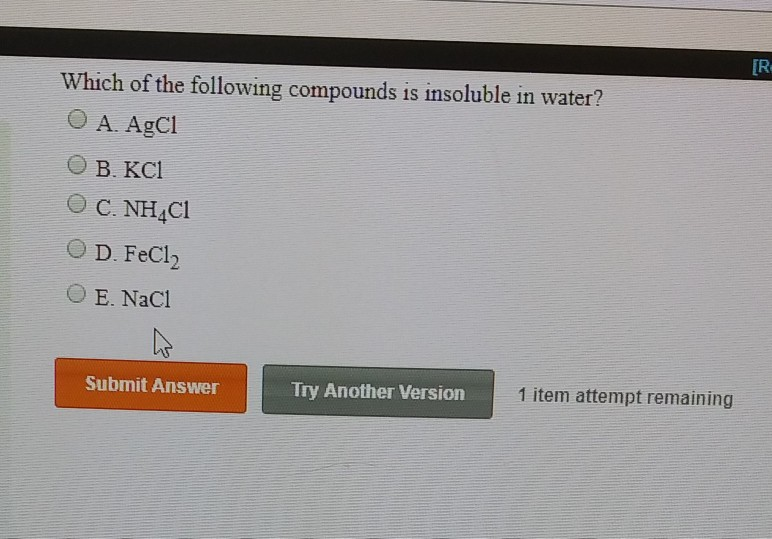
Which One Of The Following Compounds Is Insoluble In Water

Ever stood in your kitchen, staring at a pantry full of mysterious jars and boxes, and wondered, "Will this even dissolve in water?" It's a question that pops up more often than you might think, especially when you're trying to whip up something tasty or, let's be honest, just trying to clean up a sticky mess. We're talking about solubility, folks, and in simple terms, it's just how well something likes to hang out with water. Think of water as the ultimate party host, and some ingredients are just totally the life of the party, mixing in like old pals. Others? Well, they're more like the introverts of the ingredient world, preferring to keep to themselves.
So, when we're faced with a question like, "Which one of the following compounds is insoluble in water?", it's like playing a game of "who's the odd one out" at a culinary convention. We've got our usual suspects – some are famously good at mingling, others… not so much. It’s not rocket science, but sometimes it feels like it when you’re trying to figure out why your homemade gravy is looking suspiciously lumpy. That lumpiness, my friends, is often a sign that some of your ingredients are feeling a bit unwilling to join the watery shindig.
Let’s dive into this, shall we? Imagine you’re making a big batch of lemonade. You dump in the sugar, and poof! It disappears. That’s our friend, sugar (sucrose, if you want to get fancy), being a total superstar at dissolving. It’s like that one friend who knows everyone at the party and instantly makes them feel welcome. Sugar molecules are like little social butterflies, easily getting surrounded and embraced by water molecules.
Then there’s salt. You know, the stuff that makes your fries sing? Table salt, or sodium chloride, is another dissolution champion. It’s like the reliable backbone of any good flavor profile. You add it to water, and bam, it’s gone. These ionic compounds, like salt, have charged parts that water molecules, which are also a bit like little magnets, are super keen to grab onto and pull apart. It’s a match made in dissolution heaven.
But not everything is so eager to jump into the water bath. Some compounds are like those awkward guests who just stand in the corner, clutching their drink, looking decidedly out of place. They just don't vibe with water. They might be too busy having their own internal party, or maybe they're just plain repelled by water's energetic nature. These are our insoluble compounds.
The Unmixables: A Tale of Two Worlds
When we talk about insolubility, we’re talking about substances that just say, "Nah, I'm good" when water comes knocking. They prefer to stick together, like a stubborn clique at a school dance. Think about trying to mix oil and water. You can shake that bottle like you’re auditioning for a percussion solo, but no matter how hard you try, they’re just going to separate. It’s like they have a fundamental disagreement on how to spend their time. Oil molecules are nonpolar, meaning they don't have those positive and negative ends that water molecules love to latch onto. So, they just ignore each other, and eventually, the oil floats on top, looking all smug and separate.
This oil and water situation is a classic example of the golden rule of solubility: "like dissolves like." Polar solvents, like water, are great at dissolving polar solutes (like sugar and salt). Nonpolar solvents, like oil, are great at dissolving nonpolar solutes. And compounds that are very nonpolar? They’re generally not going to play nicely with our dear old water.
Now, let’s get a little more specific. When we’re presented with a list of compounds and asked to pick out the one that’s insoluble in water, we need to think about the nature of those compounds. Are they ionic? Are they polar? Are they super-duper nonpolar? It's like being a detective, examining clues to figure out who’s the shy one, who’s the social butterfly, and who’s just plain anti-social when it comes to H2O.
Decoding the Compounds: What's What?
Let's imagine a scenario. You're presented with this list (hypothetically, of course, because in real life, you’d probably have the actual names!):

- Compound A: Sodium Chloride (NaCl)
- Compound B: Potassium Nitrate (KNO3)
- Compound C: Silver Iodide (AgI)
- Compound D: Magnesium Sulfate (MgSO4)
Okay, so our mission, should we choose to accept it, is to find the one that refuses to dissolve. Let's break them down, one by one, like cracking open a fortune cookie.
Compound A: Sodium Chloride (NaCl). Ah, good ol' table salt. We already talked about this guy. He's the life of the party, the ultimate mixer. Sodium ions (Na+) and chloride ions (Cl-) are very happy to be pulled apart by water molecules. So, NaCl is definitely soluble. This one's a no-go for our insoluble list.
Compound B: Potassium Nitrate (KNO3). Potassium nitrate is often found in fertilizers and fireworks. Sounds exciting, right? Chemically speaking, it's an ionic compound. Potassium ions (K+) and nitrate ions (NO3-) are both charged. Water molecules are excellent at surrounding and separating ions. So, just like salt, KNO3 is also very soluble. Back to the drawing board for our insoluble culprit!
Compound D: Magnesium Sulfate (MgSO4). Magnesium sulfate is what you might know as Epsom salt. You might have tossed some into a bath to relax your muscles. And guess what? It dissolves! Magnesium ions (Mg2+) and sulfate ions (SO42-) are easily hydrated (surrounded by water) and dispersed. So, MgSO4 is also soluble. We’re narrowing it down!
Compound C: Silver Iodide (AgI). Now, this one's a bit different. Silver iodide is an ionic compound, but it's known for being notoriously insoluble in water. Why? Well, the attraction between the silver ions (Ag+) and the iodide ions (I-) is just too strong for water molecules to overcome easily. Think of it like a really tight hug that even energetic water molecules can’t break apart. The ions are more comfortable sticking together, forming a solid crystal lattice, rather than getting individually surrounded and pulled into the watery embrace. So, when you try to add silver iodide to water, it just sits there, like a tiny, shiny pebble at the bottom of the glass. It’s the introverted artist at the party, happy in its own little world, not keen on mingling with the masses.
The Takeaway: Why It Matters (Even If You're Not a Chemist)
So, the answer to our hypothetical question is Silver Iodide (AgI). This is because its ionic bonds are significantly stronger than the forces of attraction water molecules can exert. It’s the chemical equivalent of trying to get a grumpy cat to wear a tiny hat – it’s just not going to happen willingly!
Why should you care about this? Well, besides winning trivia nights (which is a noble pursuit, if you ask me), understanding solubility helps us in countless everyday situations. When you’re baking and a recipe calls for dissolving something, you want to know it’ll actually dissolve. Imagine trying to make a simple syrup and having your sugar clump up at the bottom! Disaster!

Think about cleaning. Why do we use soap and water? Soap is clever because it has a part that likes water (hydrophilic) and a part that likes grease and oil (hydrophobic). This allows it to grab onto greasy messes and then get washed away with the water. If grease itself dissolved in water, we wouldn’t need soap, but alas, it’s another one of those insoluble characters in the kitchen drama.
Even something as simple as taking medication involves solubility. For a drug to be absorbed by your body, it often needs to dissolve in the fluids in your stomach or intestines. If it's too insoluble, it might just pass right through, no medicinal magic happening!
So, the next time you’re dealing with a mixture, or wondering why something isn't blending in, remember the little world of solubility. Some compounds are natural-born socialites, ready to dive into water. Others are happy to keep to themselves, proving that sometimes, it’s okay to be a little bit insoluble, a little bit independent. It’s all about finding your perfect match, whether it's a molecule in a beaker or a friend at a party. And that, my friends, is a lesson we can all appreciate.
Remember that silver iodide? It's actually used in photography and as a cloud-seeding agent (to encourage rain). It’s not that it’s useless because it’s insoluble; it just has different talents. Some things are meant to mix, and some things are meant to stand their ground, looking magnificently separate. It’s the beautiful complexity of the chemical world, playing out in our kitchens, our labs, and even the skies above.
