Which Of These Statements Characterize The Nucleus Of Every Atom

Hey there, science curious friend! So, we're diving into the teeny-tiny, super-important world of atoms. You know, those fundamental building blocks of everything? From your morning coffee mug to that dazzling galaxy way out there, it’s all made of atoms. And right at the heart of every single one of these atomic superstars is something called the nucleus. Pretty cool, huh?
Now, thinking about the nucleus can get a little... well, dense, if you know what I mean. But don't worry, we're not going to get bogged down in complex equations or anything that'll make your brain do a somersault. We're just going to have a friendly chat about what definitely goes on inside that atomic command center. Think of it like this: what are the absolute, no-doubt-about-it, gotta-have-it features of every nucleus out there?
We’re going to look at a few statements, and you and I, we’re going to figure out which ones are true for every single atom’s nucleus. It’s like a little science detective mission, but way less chance of stepping on a Lego. Ready to put on your thinking cap? Or maybe just your comfy thinking slippers, that works too.
First off, let's talk about what's in there. The nucleus is essentially a bustling metropolis of two kinds of subatomic particles: protons and neutrons. Imagine them as the hardworking citizens of Atomville. Protons are the ones with a positive (+) charge, and they’re super important because they’re like the ID card of the atom. The number of protons is what tells you what element you're dealing with. So, if you've got one proton, you're hydrogen, the simplest of the bunch. If you've got 79 protons? Well, congratulations, you've got gold! Fancy, right?
Neutrons, on the other hand, are the chill ones. They have no charge. They’re like the quiet neighbors who just help keep everything stable. They don't change the element, but they can affect something called the isotope. Think of isotopes as slightly different versions of the same atom. Like how you might have a latte with whole milk, skim milk, or almond milk – it’s still coffee, but a little different. Neutrons are the reason for those variations in atoms.
So, a key characteristic of every nucleus is that it contains these protons and neutrons. All nuclei contain protons. This is a pretty solid statement. Without protons, it wouldn't even be an atom as we know it. It would just be... well, something else, and we’re talking about atoms here!
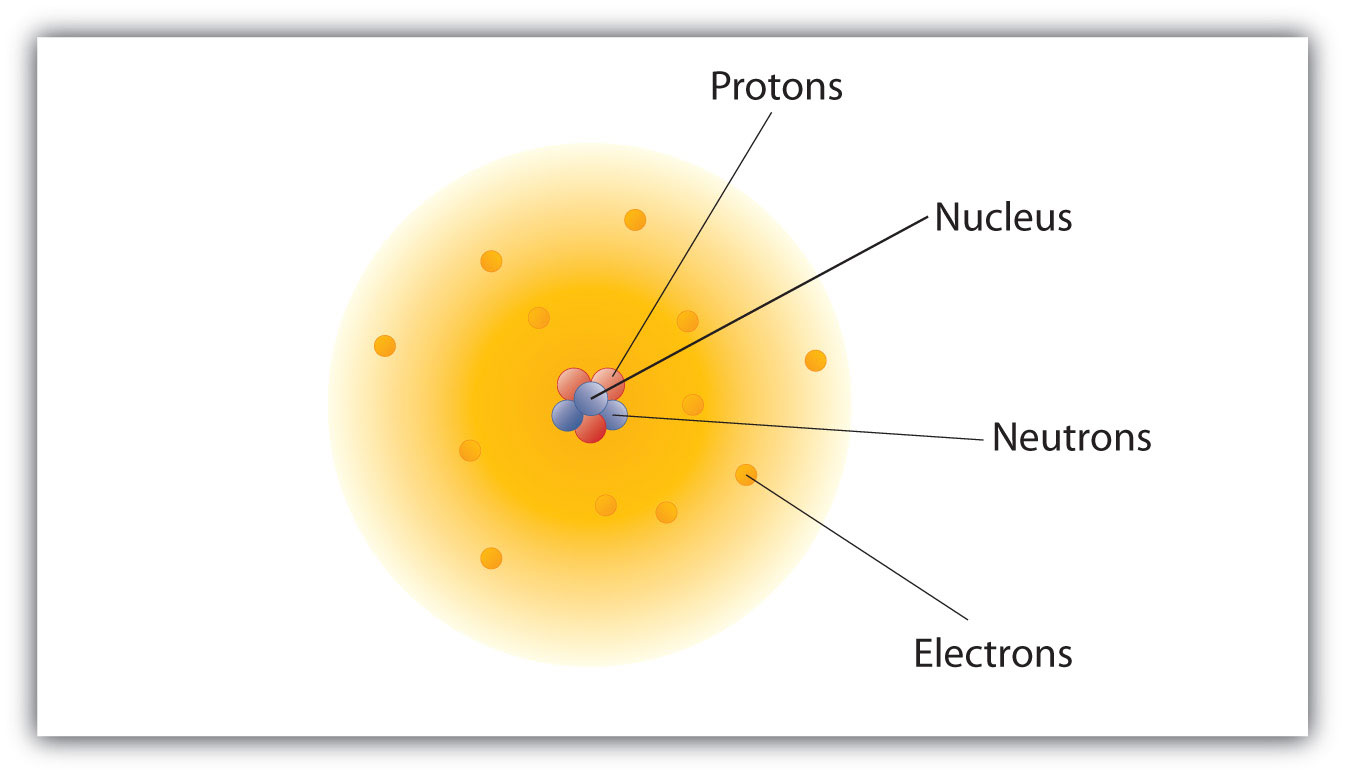
Now, what about neutrons? Do all nuclei have neutrons? This is where things get a tiny bit more nuanced, but still quite straightforward. Remember our friend hydrogen? The simplest one? Well, the most common form of hydrogen, called protium, has a nucleus with just one proton and zero neutrons. Yep, you heard that right. Just a lone proton chilling there. So, while most nuclei have neutrons, there’s that one special case. Therefore, the statement "All nuclei contain neutrons" isn't universally true. It's like saying "all dogs have fluffy tails" – sure, many do, but some have short or docked tails! So, we gotta be careful with our "all" statements.
Okay, moving on. What else can we say for sure about our little atomic cores? How about their size? Are they big and sprawling, like a city park? Or are they more like a tiny, exclusive club? Well, it turns out, nuclei are incredibly small. Like, ridiculously, mind-bogglingly small. If an atom were a sports stadium, the nucleus would be like a tiny pea in the center of the field. Seriously! Most of the atom is actually empty space. Mind. Blown.
So, is "All nuclei are incredibly small" a true statement? Absolutely! It's one of their defining features. Compared to the overall size of the atom, the nucleus is a minuscule speck. It’s like trying to find a dust mote in a hurricane – except the nucleus is the dust mote, and the atom is the hurricane. But a very, very orderly hurricane, with electrons buzzing around like tiny, frantic bees.

Now, let's talk about charge. We mentioned protons are positive and neutrons have no charge. So, what's the overall charge of the nucleus? Since protons are the only charged particles in the nucleus (neutrons are neutral), the nucleus must have a positive charge, right? If there are protons, there's positive charge. And as we established, all nuclei have protons. So, yes, every nucleus carries a positive charge. This is another biggie! It’s this positive charge that attracts the negatively charged electrons, keeping them in orbit (or, you know, in their electron clouds, it's a bit more complicated than simple orbits, but you get the gist!). It’s like a cosmic dance of opposite charges attracting.
What about density? Are these tiny nuclei packed with stuff, or are they airy and light? Oh, they are incredibly dense. Like, mind-bendingly dense. Imagine taking all the protons and neutrons from all the nuclei in your entire body and squishing them together. You'd have something so dense it would probably collapse into a black hole. Okay, maybe not that extreme, but you get the idea! The nucleus is one of the densest things in the universe. So, "All nuclei are incredibly dense" is a solid, undeniable characteristic.
Let's consider another statement: "All nuclei have a significant mass." What do you think? Protons and neutrons, while tiny, do have mass. In fact, they account for almost all of an atom's mass. Electrons are practically weightless in comparison. So, yes, it's true that all nuclei have a significant mass, at least in the context of the atom they belong to. It's where all the "stuff" of the atom is concentrated!

Now, let's try a tricky one: "All nuclei are perfectly spherical." Are they perfectly round like a billiard ball? Well, while they're generally very compact and somewhat ball-like, the shape can vary a little. It's not usually a perfectly smooth, mathematically perfect sphere. Think of it more like a slightly squishy water balloon. So, while they’re close, "All nuclei are perfectly spherical" isn't quite right. It’s a good approximation, but not a universal truth. Nature likes to keep us on our toes!
How about this: "All nuclei contain electrons"? We talked about electrons buzzing around the nucleus, right? They're like the entourage of the atomic VIP. But are they inside the nucleus? Nope! Electrons hang out outside the nucleus. They're attracted to the positive charge of the protons, but they don't get to party inside the nucleus itself. So, the statement "All nuclei contain electrons" is a big fat false. Electrons are the outer circle, the nucleus is the core!
Let's recap what we've figured out are the universal truths about the nucleus:

The Undeniable Truths About Every Atom's Nucleus:
- All nuclei contain protons. This is non-negotiable. Protons are the fingerprint of an element.
- All nuclei have a positive charge. Thanks to those lovely protons!
- All nuclei are incredibly small. Relative to the atom, they’re microscopic.
- All nuclei are incredibly dense. Packed tighter than a can of sardines!
- All nuclei have a significant mass. They’re the heavyweight champions of the atom.
And what we've learned is not universally true for all nuclei:
- Not all nuclei contain neutrons. Shoutout to our minimalist hydrogen friends!
- Not all nuclei are perfectly spherical. They’re more like slightly lumpy marbles.
- No nuclei contain electrons. Electrons are the cool kids hanging out on the outside.
Isn't it fascinating how much we can learn just by thinking about what’s always true for something so fundamental? It’s like understanding the core principles of friendship: everyone needs someone to talk to (protons!), and it helps to have someone to keep you grounded (neutrons, usually!). And even though the nucleus is tiny, it holds immense power and defines the very essence of what something is. It’s the concentrated power, the heart and soul of the atom.
So, the next time you look at anything – your hand, a tree, a distant star – remember that at its very core, it's all made of these amazing, tiny, powerful nuclei. They are the architects of reality, the hidden engines of the universe. And even though they're too small to see, their presence is what makes everything possible. Pretty amazing, right? Keep exploring, keep wondering, and keep that brilliant mind of yours buzzing with curiosity! The universe is a vast and wonderful place, and you're a part of its incredible story.
