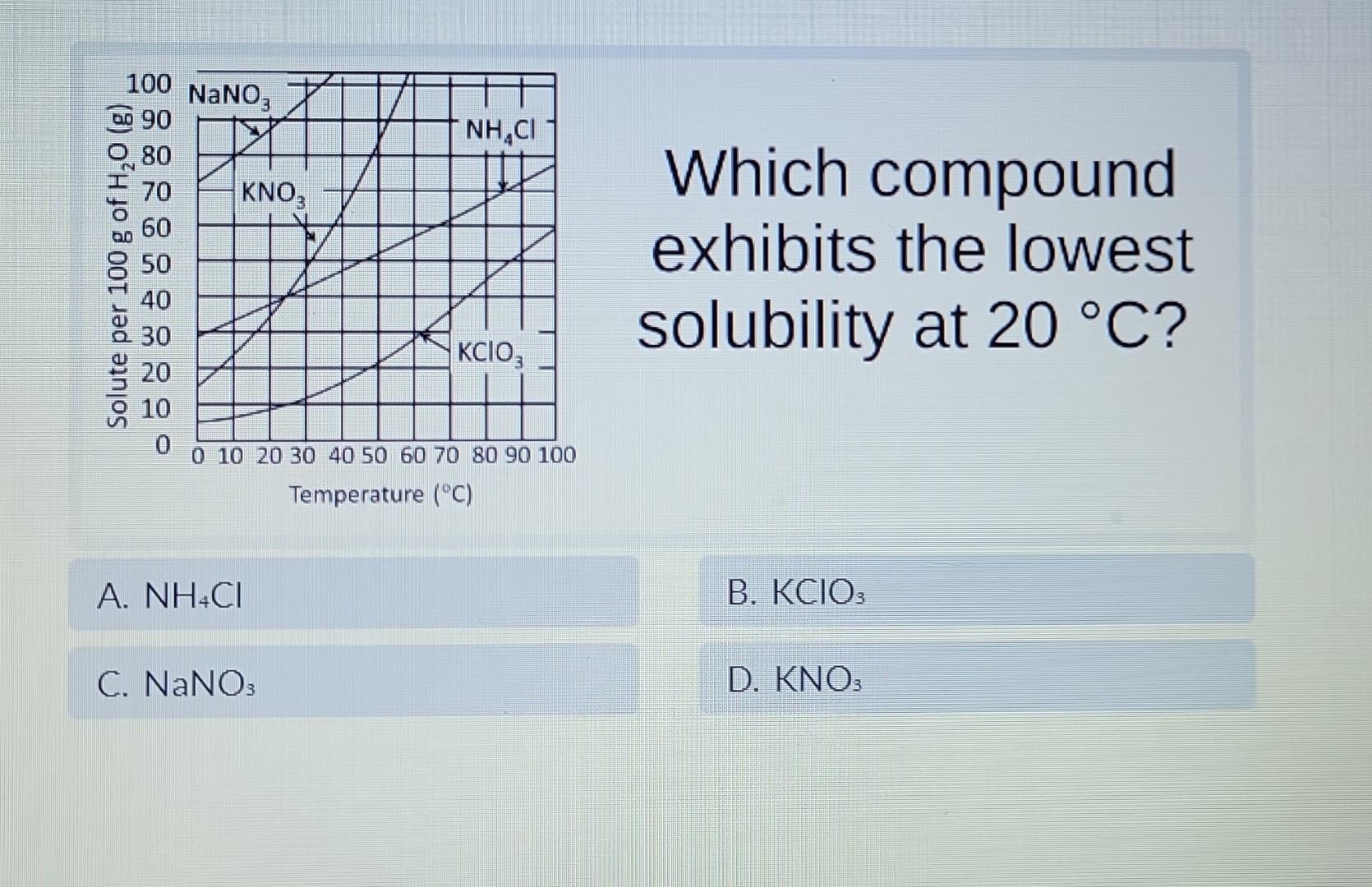
Which Of The Samples Most Likely Had The Lowest Solubility
Alright, pull up a chair, folks, and grab a cuppa because we're about to dive headfirst into the thrilling, the mystifying, the occasionally sticky world of… well, samples and their solubility. Now, before you nod off thinking this sounds like a particularly dry lecture from that one professor whose tie always seemed to be plotting against his shirt, let me assure you, this is more like a CSI episode, but with less dramatic music and significantly more beakers.
We've been tinkering, we've been stirring, and we've been staring intently at various concoctions, trying to figure out which of our brave little sample soldiers decided to stage a silent protest when it came to dissolving. Think of it as a talent show for molecules, and some of them are just not cut out for the solo spotlight in a solvent sea.
The Case of the Stubborn Solutes
So, picture this: we had a lineup of suspects, each with their own unique personality. We’ve got Mr. Water-Lover, Ms. Oil-Appreciator, and then, lurking in the shadows, we have our prime suspect. This particular character seemed to have a deep-seated aversion to anything remotely watery. Like a cat at a bath convention, it just… wouldn’t.
We started with our usual suspects, the trusty water-based solvents. You know, H2O, the universal solvent, the stuff that keeps us all hydrated and occasionally makes our coffee taste like, well, coffee. We tossed in our samples, gave them a good swirl, and waited with bated breath. Some went in with a cheerful fizz, like tiny champagne corks popping. Others dissolved with a whisper, disappearing like a ninja in the night. And then there was him.
This one sample, let’s call it… the Great Wall of China for dissolving. It just sat there. Unmoved. Unbothered. It was like trying to convince a toddler to eat broccoli. You can prod, you can plead, you can even sing songs about it, but it’s going to remain resolutely itself. We tried warming it up, thinking maybe a little heat would loosen it up. Nope. We tried shaking it with the fury of a thousand angry bees. Nada. It was a true enigma, a scientific Houdini who refused to escape the clutches of its solid form.
Unmasking the Culprit
Now, how do we know which one was the least soluble? It's all about what didn't happen. For most of our samples, we saw them gracefully merge with the solvent. They became one, a beautiful molecular love story. But our star player? It remained stubbornly separate. Imagine you’re at a party, and everyone’s mingling, dancing, having a grand old time, and then there’s that one person, plastered against the wall, clutching their drink, looking like they’d rather be anywhere else. That was our sample.
We saw distinct layers forming. The solvent was happily chilling on one side, and our sample was doing its own thing on the other, like they were in a silent, passive-aggressive standoff. It was less of a solution and more of a cohabitation of inconvenience.
So, what makes a molecule play hard to get with a solvent? Well, it’s all about their personalities, their chemical personalities. Think of it like dating. Some molecules are like magnets, attracted to water's polar nature. They're the social butterflies, the extroverts of the molecular world, happy to dive into any aqueous adventure. These are our hydrophilic little buddies – “water-loving,” if you want to get fancy.

But then you have the other guys. The rebels. The ones who prefer the company of oily, greasy things. They’re the introverts, the loners, who find water a bit… much. They’re hydrophobic, meaning “water-fearing.” And our least soluble sample? It was practically a phobic when it came to water. It was like trying to get oil and water to become best friends, which, as we all know, is about as likely as finding a parking spot downtown on a Saturday night.
A Tale of Two Bonds
The real magic, or lack thereof, lies in the bonds between the molecules. Water molecules are like tiny little magnets, with a positive end and a negative end. They love to cling to other molecules that have a similar magnetic attraction, pulling them apart and dissolving them. This is called polarity.

Our hydrophobic sample, however, was likely made up of molecules that were more uniform in their charge distribution. They didn’t have those distinct positive and negative poles. They were like little bowling balls, smooth and uncharged, and water just couldn’t get a good grip on them. They preferred to stick together, forming a solid mass, because, hey, at least they understood each other.
Think of it this way: imagine trying to dissolve a Lego brick in a swimming pool. It’s just not going to happen, right? That Lego brick is a solid, cohesive unit, and the water molecules can't break it down. Our least soluble sample was essentially our scientific Lego brick, resisting the watery embrace with all its might.
Another clue we look for is the size and shape of the molecules. Sometimes, even if a molecule has some slight attraction to a solvent, if it's too big or awkwardly shaped, it just can’t fit into the solvent’s molecular dance. It’s like trying to cram a king-sized mattress through a cat flap. It’s physically impossible. So, our stubborn sample might have been a bit of a molecular behemoth, or perhaps it had a shape that just didn't play well with others in the solvent pool.

The Smoking Gun (or Beaker)
So, when we look at our results, the sample that remained largely as a separate, solid entity, even after vigorous stirring and perhaps a little bit of pleading, is our undisputed champion of insolubility. It showed us that not all substances are created equal when it comes to dissolving. Some are ready to mingle, others are content to be wallflowers, and then there are those who flat-out refuse to leave their comfort zone, no matter how much you offer them a fancy drink.
The surprise here isn't that something didn't dissolve, it's the degree to which it refused. It was a masterclass in molecular stubbornness. We saw samples that dissolved partially, leaving a cloudy suspension, like a slightly disappointing snow globe. But this one? This one was the pure, unadulterated, “I’m not going anywhere” kind of insoluble. It was the silent protest we'd been looking for.
It's a humbling reminder that nature operates on its own set of rules, and sometimes, those rules involve molecules deciding they'd rather stick together than get dissolved. And honestly, sometimes, we can all relate to that feeling, right? Pass the biscuits, will you?
