Which Of The Following Substances Dissolves Most Readily In Gasoline
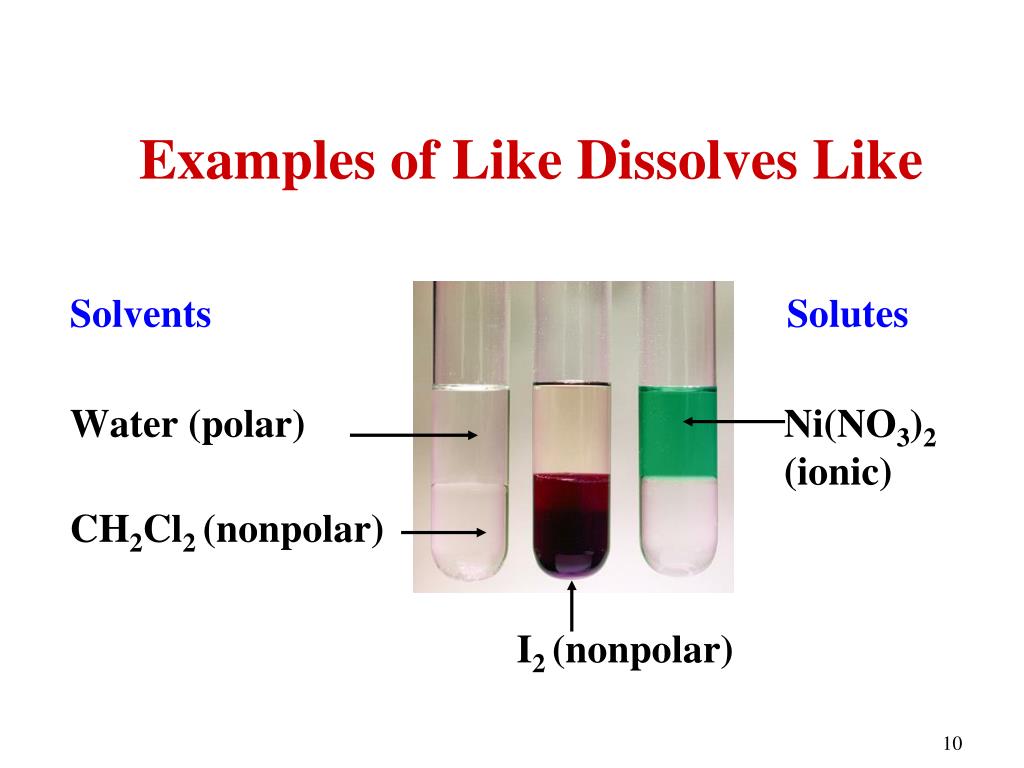
Hey there, fellow curious minds! Ever find yourself staring at a can of gasoline and wondering, "What else can this stuff dissolve?" It's kind of like that scene in a sci-fi movie, right? You've got this super-powerful solvent, and you just know there are secrets hidden within its murky depths. Well, today, we're going on a little adventure to explore just that. We're diving into the fascinating world of solubility, and we've got a few suspects lined up to see who plays nicely with gasoline. Get ready to be a little bit amazed, and maybe even a tad bit impressed by our good ol' friend, gasoline!
So, why is this even interesting, you ask? Think about it! Gasoline is pretty much everywhere, powering our cars, our lawnmowers, even some of those fancy portable generators. Understanding what it can and can't dissolve is not just for chemists in lab coats. It's practical stuff! It helps us understand how things might get cleaned (or, uh oh, ruined) if they come into contact with it. Plus, it’s a super cool way to peek into the tiny, invisible world of molecules and how they interact.
Imagine you have a big, messy painting, and you’re looking for the perfect eraser. Some erasers are gentle and only get rid of pencil marks. Others are so powerful they might accidentally smudge the whole picture! That’s kind of what we're talking about with solvents and solutes – the stuff being dissolved. Gasoline is a pretty strong solvent, meaning it's good at breaking things down. But what kind of "things" does it break down best?
We've got a few contenders lined up for our solubility showdown. Let's meet our contestants:
Our Solubility Contestants!
First up, we have water. The universal solvent, right? We drink it, we bathe in it, it's essential for life. But how does it get along with gasoline? Not so well, as it turns out!
Then we have salt. You know, table salt. The stuff that makes your fries taste amazing. It dissolves in water like a dream. But will it dissolve in gasoline? Let's see.

Next, we're bringing in sugar. Another kitchen staple! Sweetens our coffee, makes cookies possible. Like salt, it loves to hang out with water. What about gasoline?
And finally, we have something a little more, shall we say, related to gasoline: oil. Like vegetable oil, or even the oil you might use to lubricate a squeaky hinge. This is where things get interesting, because gasoline itself is a petroleum product.
So, the big question is: which of these will vanish into gasoline the quickest and most completely? It’s like a race to see who can disappear the fastest!

The Great Dissolving Race Begins!
Let's start with water. You've probably seen this happen. If you’ve ever accidentally mixed water with gasoline, what happens? Do they blend together like a smooth milkshake? Nope! They stubbornly refuse to mix. They separate into distinct layers, with the gasoline floating on top. This is because water molecules are polar, and gasoline molecules are non-polar. Think of it like trying to mix oil and vinegar – they just don’t want to be friends. So, water is definitely not the winner here.
Now, let's talk about salt. You know how salt dissolves in water? It's like the water molecules grab onto the salt ions and pull them apart. But gasoline, remember, is non-polar. It doesn't have those charged parts that can attract and break down the ionic bonds in salt. So, if you dump salt into gasoline, it’s just going to sit there, like tiny white pebbles at the bottom of the can. Definitely not dissolving readily.
What about sugar? Sugar also loves water. It’s made of molecules that have parts that can interact with water molecules. But like salt, gasoline’s non-polar nature isn't very good at attracting and dissolving sugar molecules. So, your sugar cubes will remain stubbornly solid, just like the salt. No sweetening of the gasoline, I'm afraid.
This brings us to our final contestant: oil. Now, this is where the magic happens, or at least, where the dissolving happens. Remember how we said gasoline is a petroleum product? That means it's made up of a bunch of hydrocarbons – long chains of carbon and hydrogen atoms. Many types of oils, like vegetable oil or motor oil, are also made up of hydrocarbons. They have similar molecular structures.

Think of it like this: if you have a bunch of Lego bricks that are all the same shape and size, they're going to fit together really well. Gasoline molecules and oil molecules are like those matching Lego bricks. They have a similar "liking" for each other, and they can easily intermingle and spread out amongst each other.
So, when you mix oil with gasoline, they actually do dissolve! They blend together, forming a uniform mixture. This is because of a fundamental principle in chemistry: "like dissolves like." Non-polar solvents, like gasoline, are really good at dissolving non-polar solutes, like oil. Polar solvents, like water, are good at dissolving polar solutes, like salt and sugar.
The Champion Revealed!
Drumroll, please! 🥁 The substance that dissolves most readily in gasoline is, without a doubt, oil.

Isn't that neat? It makes so much sense when you think about it. Gasoline is essentially a lighter, more volatile form of oil. They’re practically cousins in the molecular world! This is why, for example, you might use a bit of gasoline to help clean greasy or oily stains off certain surfaces (though, always with extreme caution and in a well-ventilated area, of course!). The gasoline can break down and carry away the oil you're trying to remove.
It's like having a bouncer at a club. A bouncer who only lets in people who look and act like the other people already inside. Gasoline is the bouncer, and oil is invited because it fits the vibe. Water, salt, and sugar? They're wearing the wrong clothes and have a different kind of energy, so they're politely (or not so politely) shown the door.
So, the next time you’re near a can of gasoline, you can impress your friends with your newfound knowledge of solubility. You can explain that while water, salt, and sugar are all good friends with each other, they’re not exactly BFFs with gasoline. But oil? Oh, oil and gasoline are basically inseparable!
It’s a small piece of the puzzle that makes up our world, but understanding these little interactions can be surprisingly satisfying. It’s a reminder that even seemingly simple substances have complex and fascinating behaviors. Keep asking questions, keep being curious, and you'll discover something cool every single day!
