Which Of The Following Steps In Solution Formation Is Exothermic
Hey there, my fellow science enthusiasts and anyone who's ever wondered why dissolving stuff can sometimes feel like a tiny magical trick! Today, we're diving into something super interesting: solution formation. Now, I know what you might be thinking, "Solution formation? Is that going to involve complicated equations and looking confused in a lab coat?" Absolutely not! We're going to keep this super chill, like we're just hanging out and chatting about how things mix together. Think of it like making a delicious cup of tea or stirring sugar into your coffee – simple, right? But there's some cool science happening behind the scenes, and we're going to unpack it in a way that's as easy as, well, dissolving something in water!
So, what is a solution, anyway? Basically, it's when you have two or more substances that mix together so perfectly, they become one single, uniform phase. Think of that sugar you stirred into your tea. It doesn't just sit there in clumps (unless you're going for that extra crunchy texture, which, no judgment!). It disappears, spreading out evenly. That's a solution! One part is called the solute – that's the stuff that gets dissolved (like our sugar). The other part is the solvent – that's the stuff that does the dissolving (like our tea, or more scientifically, often water).
Now, the big question we're tackling today is: Which of the following steps in solution formation is exothermic? Woah there, hold your horses! Before you start breaking out your flashcards, let's break down what "exothermic" even means. Think of it like this: exo sounds like "exit," right? And thermic is about heat. So, exothermic means heat is exiting the system, or in simpler terms, heat is released. When something is exothermic, it means the process gives off heat, and you can often feel it getting warmer. Think of a hand warmer on a cold day – that's usually an exothermic reaction!
On the flip side, you've got endothermic processes. These guys absorb heat from their surroundings. So, if you mix something endothermic, the container might feel a little cooler. Remember those instant cold packs you can crack open for an injury? That's a classic example of an endothermic reaction – it feels cold because it's sucking up all the heat around it!
Alright, so solution formation isn't just one single "poof, it's dissolved!" moment. It's actually a series of steps, and each step has its own energy story. Imagine you're trying to get a bunch of people (solute particles) to mingle and make friends with a whole bunch of other people (solvent particles). It's not always a smooth party, you know?
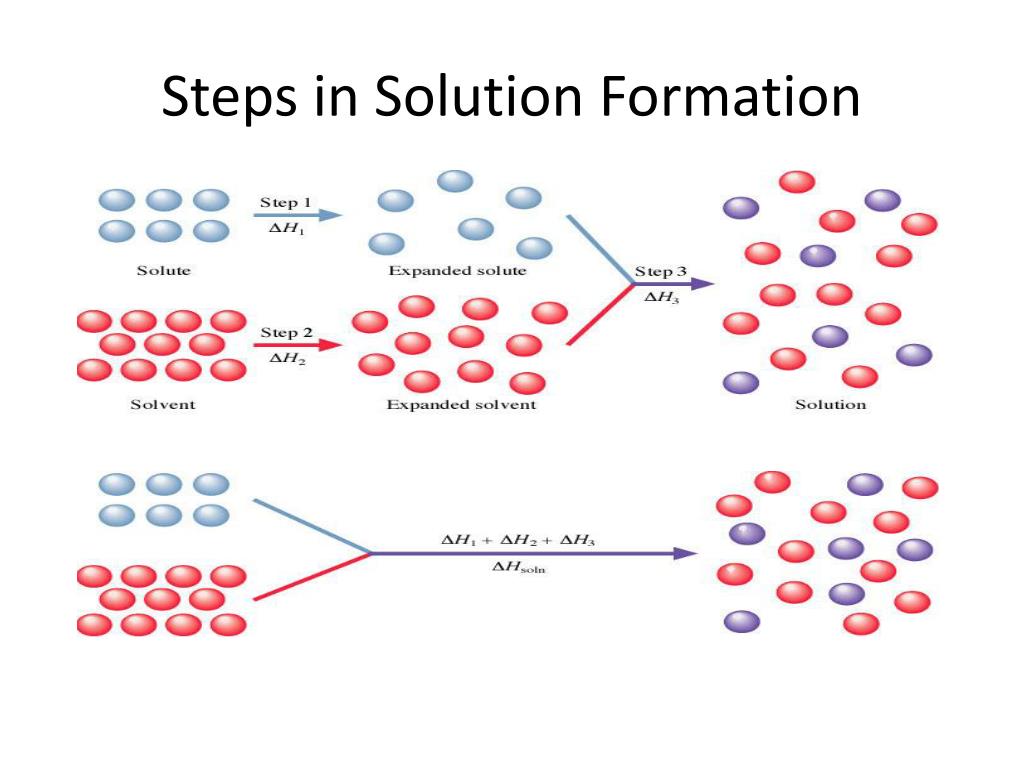
The process of forming a solution can be broken down into three main steps. And guess what? We're going to explore each one like a detective looking for clues to where the heat is hiding (or being released!).

Step 1: Breaking Up is Hard to Do (Solute-Solute Interactions)
Our first step is all about the solute particles. Before they can even think about mingling with the solvent, they've got to loosen their grip on each other. Imagine our solute particles are like little magnets that are really, really attracted to each other. To pull them apart, to get them ready to meet new friends, you need to put in some energy, right? You have to overcome those attractions.
This step is like convincing your best friends to leave their comfy couch to go to a party. You need to put in some effort, some energy, to persuade them. Because you're breaking attractive forces, this step almost always requires energy to be put in. This means it's an endothermic process. We're putting energy in to separate them. Think of it as the "getting ready" phase, and sometimes that involves a bit of effort and a bit of heat absorption to loosen things up. So, if you're imagining a thermometer near this step, it would be showing a slight dip in temperature.
Step 2: Making Some Space! (Solvent-Solvent Interactions)
Now, the solvent particles also need to make some room for the incoming solute guests. Imagine our solvent particles are also holding hands, enjoying their own little solvent party. To make space for the solute particles to slide in, the solvent particles have to move apart a bit. Again, this involves overcoming the attractions between solvent molecules. Think of it like pushing people aside slightly on a dance floor to make space for new dancers.
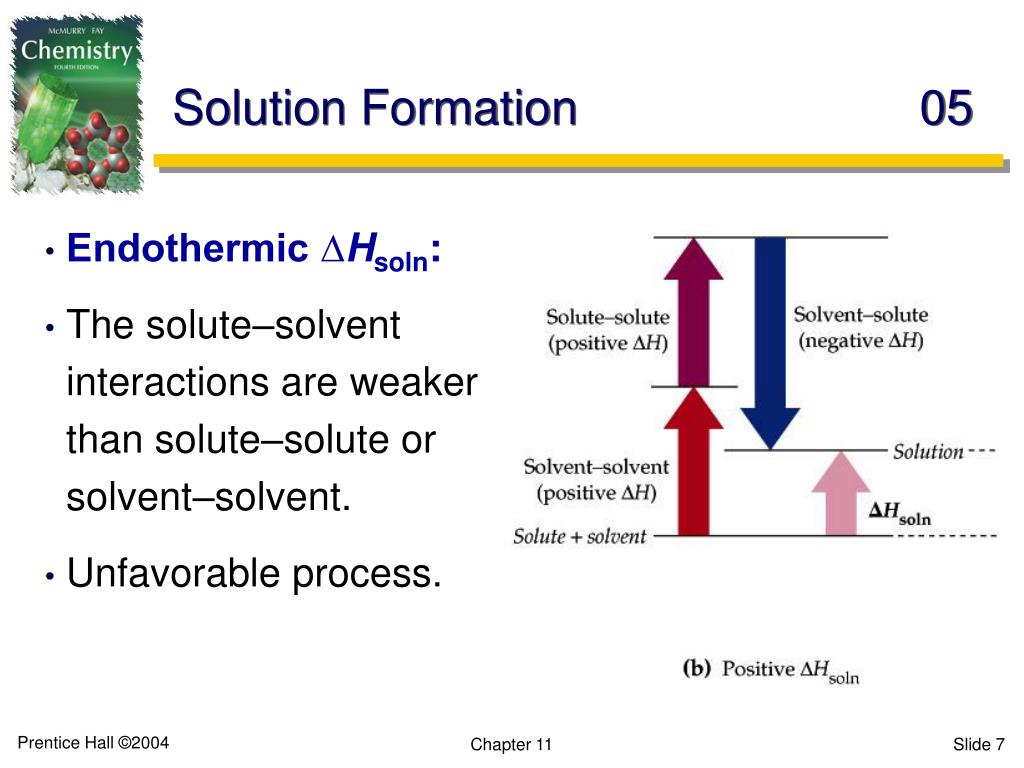
Just like with the solute, breaking these solvent-solvent attractions also requires energy to be put in. So, this step is also typically endothermic. We're putting energy in to create gaps, to make room for the solute to infiltrate. It's like preparing the venue for the big mingle. Again, a thermometer here would likely show a slight cooling effect as energy is absorbed to create these spaces.
Step 3: The Grand Introduction (Solute-Solvent Interactions)
Ah, the moment of truth! This is where the solute particles and the solvent particles finally meet and start forming new attractions. Imagine our solute particles, now separated and ready, bumping into the solvent particles, who have also made some space. When they get close, they start to interact, and new attractive forces are formed between the solute and solvent molecules.
Now, here's the exciting part! When new attractive forces are formed between different types of molecules (solute and solvent), energy is released. Think of it like a happy reunion! When these new bonds are made, they are often more stable than the original bonds that were broken in steps 1 and 2. This release of energy is what we're looking for. This step is typically exothermic. Heat is released as the solute and solvent happily embrace each other, forming those beautiful new interactions.
So, Which Step is Exothermic?
Putting it all together, if we look at those three steps: breaking solute-solute bonds (endothermic), breaking solvent-solvent bonds (endothermic), and forming solute-solvent bonds (exothermic), it's pretty clear where the heat is coming from, right?
The step where new attractions are formed between the solute and solvent particles is the one that is generally exothermic. This is where the energy is released, and it's the crucial step that makes the overall process of solution formation contribute to the heat balance of the system.
Now, it's important to remember that the overall heat change for solution formation (called the enthalpy of solution) depends on the balance of energy changes in all three steps. Sometimes, the endothermic steps are a little stronger, and the overall process can still be endothermic (making things feel cooler). Other times, the exothermic step is so powerful that it outweighs the energy needed for the other two, and the overall process is definitely exothermic (making things feel warmer!).

Think about dissolving ammonium nitrate in water. That's what makes those instant cold packs work! The energy needed to break the solute-solute and solvent-solvent bonds is greater than the energy released when the ammonium nitrate and water molecules interact. So, the overall process is endothermic, and it feels cold. On the other hand, dissolving a strong acid like sulfuric acid in water can be quite dramatic and release a lot of heat – it's very exothermic!
The Takeaway Message
So, while the overall enthalpy of solution can vary, the specific step that is fundamentally exothermic is the one where solute and solvent particles form new attractive forces. It's like the "making friends" part of the process, where the energy is released as these new connections are solidified.
Isn't that neat? It's like a mini chemical dance, with energy being absorbed to break old partnerships and then released as new, exciting ones are formed. It’s a beautiful illustration of how energy plays a role in bringing things together. So next time you're dissolving something, you can imagine those tiny particles getting ready, saying goodbye to their old buddies, and then joyfully embracing their new solvent friends, releasing a little bit of that celebratory warmth. Keep exploring, keep wondering, and remember that even the simplest acts of mixing can hold a world of fascinating science!
