Which Of The Following Statements Regarding Lipids Is True
Hey there, you! Grab your coffee, let's chat. Ever stared at a biology textbook, feeling like you're trying to decode an alien language? Yeah, me too. Especially when we get to the nitty-gritty of, like, what is a lipid, anyway? It sounds so… scientific. But honestly, it's not that scary. Think of lipids as the unsung heroes of our cells. The ones doing all the heavy lifting, the structural work, the energy storage. You know, the stuff that keeps everything from falling apart. And let's be real, who doesn't love a good, greasy chip? That's where lipids come in! They're not just boring molecules, they're the reason we can even have delicious snacks. So, let's dive in, shall we? We're gonna tackle a classic question: Which of the following statements regarding lipids is true? Sounds like a pop quiz, right? But trust me, it's more like a fun little brain teaser. We're not aiming for perfect scores here, just understanding. Because understanding how our bodies work, even the tiny, wiggly bits, is pretty darn cool.
So, let's get down to business. We've got a bunch of statements about lipids, and we need to pick the one that's actually, you know, real. Like, the factual one. The one that makes sense. The one that a biologist would nod enthusiastically at. It’s like finding the perfectly ripe avocado – you just know when it’s right. So, first things first, what even are lipids? Are they like, tiny little building blocks? Or more like… the glue that holds everything together? The answer, my friend, is a bit of both, and then some! They're a diverse bunch, these lipids. You've got your fats, your oils, your waxes, and even some of the stuff that makes up your brain. Wild, right? Your brain is practically a lipid factory!
Let’s break down some of the usual suspects you might see in these kinds of questions. Often, they’ll try to trick you by mixing up lipids with other types of molecules. Like, “Lipids are a type of carbohydrate.” Uh, no. Carbohydrates are like, sugar, starch – the quick energy providers. Lipids are more like the long-term savings account for your energy. They pack a bigger punch per gram, which is why that extra slice of cake can sometimes feel like it’s sticking around for a while. And then there’s the classic confusion with proteins. Proteins are the workers. They do everything from building muscles to carrying oxygen. Lipids are more about the structure and the storage. Different jobs, you know?
Now, let’s get specific. One statement you might encounter is something like: “All lipids are soluble in water.” This is where we start to separate the edible from the… well, not-so-edible in terms of solubility. Think about oil and water. Do they mix? Nope. They just sit there, doing their own thing. This is because lipids are hydrophobic. That's a fancy word for "water-fearing." They don't like to hang out with water molecules. Water molecules are polar, meaning they have a bit of a positive and a bit of a negative side, and they love sticking to each other. Lipids, on the other hand, are mostly nonpolar. They’re like the lone wolves of the molecular world, preferring to associate with other nonpolar things, like, you guessed it, other lipids!
So, if that statement about all lipids being soluble in water is floating around, you can probably wave it goodbye. It's a bit of a red herring, if you ask me. It’s like saying all birds can fly. What about penguins? Or ostriches? They're birds, but they’re not exactly soaring through the sky. Similarly, there are some exceptions, but the general rule is that lipids are not soluble in water. They’re more at home in organic solvents, like alcohol or acetone. Ever tried to get grease out of a shirt? You usually need something a bit stronger than just water, right? Soap works because it has parts that like water and parts that like grease, acting as a bridge. But that’s a whole other tangent we could go down!
Another statement that might pop up is something along the lines of: “Lipids are exclusively used for energy storage.” Now, this one sounds plausible, right? We know we store fat for energy. But is that all they do? Absolutely not! That would be like saying your smartphone is only good for making calls. It does so much more! Lipids are fundamental to cell structure. Think about the cell membrane. What’s that made of? Yep, a bilayer of phospholipids. Phospholipids are a type of lipid, and they form the outer boundary of every single cell in your body. They’re like the little bouncers, controlling what goes in and out. Pretty crucial role, wouldn't you say?
And it doesn't stop there. Some lipids are involved in cell signaling. They act as messengers, telling cells what to do and when to do it. Hormones like estrogen and testosterone? They're steroids, and steroids are a type of lipid! So, those little molecules are pretty darn important for, you know, lots of things. Growth, development, reproduction – the whole shebang. So, if a statement claims lipids are exclusively for energy storage, you can probably cross that one off your list too. They're multi-talented performers, these lipids.

Okay, so what else might they throw at us? Sometimes, they’ll get a bit more specific, which can be helpful or, you know, just more confusing. For instance, you might see: “Triglycerides are the primary form of energy storage in plants and animals.” Now, this one is getting warmer! Triglycerides are the main type of fat we think of. They’re made of a glycerol molecule and three fatty acid chains. And yes, they are indeed the primary way both plants and animals store energy. Plants store them in seeds (think sunflower seeds, your favorite!), and animals store them in adipose tissue (that's just a fancy word for fat tissue, where your body keeps its energy reserves). So, this statement has a lot of truth to it.
But is it the most true statement among a set of choices? That's the million-dollar question. Sometimes, even a true statement can be overshadowed by one that's more comprehensively true or addresses a broader characteristic of lipids. It’s like choosing between saying “This apple is red” and “This apple is a fruit.” Both are true, but “This apple is a fruit” is a more fundamental classification. So, while the triglyceride statement is good, we need to keep our eyes peeled for something that defines lipids more broadly or addresses a characteristic that’s universally true for all lipids (or at least the vast majority of them in a defining way).
Let’s think about the structure again. Lipids are defined by their solubility characteristics, not necessarily by a specific functional group like carbohydrates or proteins have. Remember that hydrophobic thing? That's a biggie. So, a statement that centers on their interaction with water and their nonpolar nature is usually a strong contender for being the true statement.
Consider this possibility: “Lipids are a diverse group of naturally occurring organic compounds that are generally insoluble in water but soluble in nonpolar organic solvents.” How does that sound? Pretty solid, right? It covers their diverse nature, their key solubility characteristic (the hydrophobic part), and where they do dissolve. This statement encompasses fats, oils, waxes, steroids, and phospholipids. It’s a broad, accurate definition. It doesn’t limit them to just one function, nor does it make absolute claims about solubility that might have a rare exception. It speaks to their fundamental chemical nature.

Let’s ponder another statement that might be thrown into the mix: “All fatty acids contain at least one double bond.” This is where we need to remember the difference between saturated and unsaturated fatty acids. Saturated fatty acids have no double bonds between the carbon atoms in their chain. They are “saturated” with hydrogen atoms. Think of them as being very tightly packed. Unsaturated fatty acids, on the other hand, do have at least one double bond. This makes them a bit more flexible, and they tend to be liquid at room temperature (like olive oil). So, if a statement says all fatty acids have a double bond, that's a big fat nope. It’s like saying all dogs are golden retrievers – clearly not true!
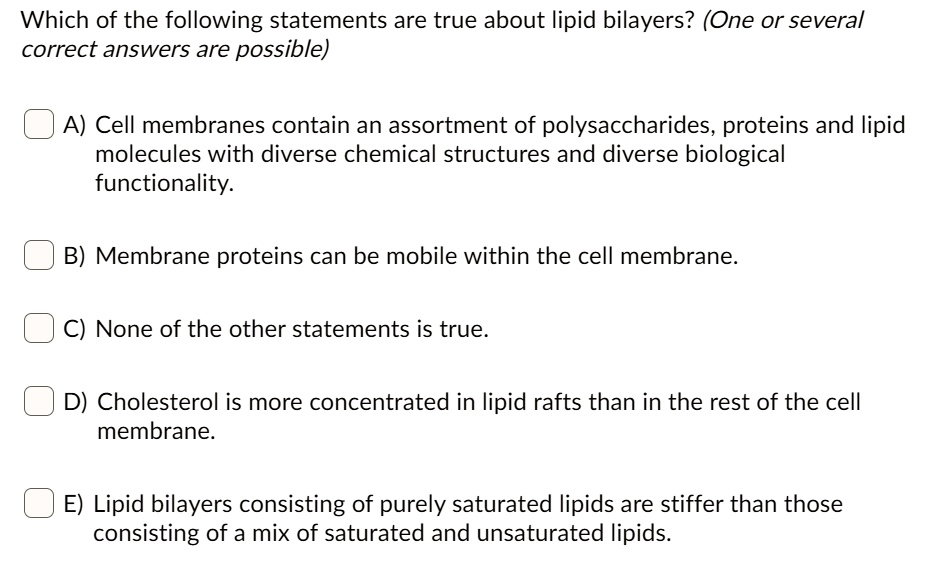
So, when you're faced with this kind of question, it's all about process of elimination and understanding the core properties. You look at each statement and ask yourself: 1. Is this always true for lipids? 2. Is this too specific and only applies to certain types of lipids? 3. Does this contradict a fundamental characteristic of lipids (like solubility)?
Let’s imagine a hypothetical scenario. You’re given these options:
A) Lipids are composed solely of carbon, hydrogen, and oxygen, with a 2:1 ratio of hydrogen to oxygen.
![[ANSWERED] Which of the following statement regarding lipid lin](https://media.kunduz.com/media/sug-question-candidate/20231023225014565355-4412073.jpg?h=512)
B) Lipids are polar molecules that readily dissolve in water.
C) Lipids are a class of compounds characterized by their insolubility in water and solubility in organic solvents.
D) All lipids function exclusively as structural components of cell membranes.
Let’s break these down. Option A? That ratio of hydrogen to oxygen (2:1) is the hallmark of carbohydrates. Lipids have a much higher proportion of hydrogen compared to oxygen. So, A is out. Definitely out. It’s a carbohydrate giveaway!
Option B? We already talked about this! Lipids are hydrophobic. They don't readily dissolve in water. So, B is also a goner. Think oil and water again. Still not mixing!
Option D? We know lipids do way more than just be structural components of cell membranes. They store energy, they’re involved in hormones, signaling… the list goes on. So, D is too limiting. It’s like saying humans only exist to breathe. We do a bit more than that!
Now, let’s look at Option C: “Lipids are a class of compounds characterized by their insolubility in water and solubility in organic solvents.” Ding ding ding! This is the winner! It accurately describes their fundamental chemical behavior. It acknowledges that they are a class of compounds, implying diversity, but focuses on the unifying characteristic that defines them as lipids: their interaction with polar (water) and nonpolar (organic solvents) substances. It’s the most encompassing and factually accurate statement.
So, when you see a question like "Which of the following statements regarding lipids is true?", don't panic. Just channel your inner scientist, think about those greasy fries, that protective layer of wax on a leaf, and the very building blocks of your cell walls. They’re all lipids, and they’re all doing their own unique thing. But the common thread? That hydrophobic nature. That’s the key. It’s like a secret handshake for lipids. They might be diverse, they might have different jobs, but they all tend to shy away from water and buddy up with other nonpolar molecules. So, next time you're munching on something fatty, give a little nod to the incredible world of lipids. They're working hard, even when you're just relaxing!
Remember, biology is all about patterns and characteristics. Lipids are defined by a set of properties, and their solubility is a major one. While there can always be nuanced exceptions in complex biological systems, for the purpose of a general question like this, you're looking for the statement that best captures the defining features of the entire group. It's about recognizing the general, overarching truth. And in the world of lipids, that truth is inextricably linked to their relationship with water. They’re the hydrophobic buddies of the cell, and that’s what makes them so special… and delicious in our food!
