Which Of The Following Statements Concerning Saturated Fats Is True

Hey there, friend! So, we're diving into the fascinating (and sometimes confusing!) world of fats. Specifically, we're going to tackle a question that might pop up on a quiz, or maybe you're just curious about what's what in the land of lipids. The big question is: Which of the following statements concerning saturated fats is true?
Now, before your eyes glaze over thinking about chemistry class, let's make this super chill. We're going to break it down like we're chatting over coffee (decaf, of course, if we're being really careful about our fat intake, haha!).
First off, what exactly are saturated fats? Think of them like a tightly packed suitcase. Each carbon atom in the fat molecule is holding hands with as many hydrogen atoms as it possibly can. It's completely "saturated" with hydrogen. It's like a super stable, happy little molecule that doesn't need to bond with anything else. Hence, "saturated." Pretty straightforward, right? They're typically solid at room temperature, like butter or the fat on a steak. You know, the stuff that makes your fry-up extra delicious (but maybe not super good for you in large quantities, more on that later!).
So, What's the Big Deal with Saturated Fats?
This is where things get a little more complex, and where some of those true/false statements might come into play. For ages, saturated fats were basically the villain of the nutritional world. We were told to avoid them like a bad date. And for good reason, in many cases. Too much saturated fat has been linked to an increase in "bad" cholesterol (LDL cholesterol), which can clog up your arteries. Think of your arteries as busy highways, and LDL cholesterol as slow-moving traffic jams. Not ideal for smooth sailing, right?
But here’s the fun twist, and where the science gets a bit more nuanced: not all saturated fats are created equal! This is a key point that often gets lost in translation. Just like not all people named Bob are the same (some are awesome, some… well, you get it), different types of saturated fats can have different effects on our bodies.
Some saturated fatty acids are short-chain, some are medium-chain, and some are long-chain. And guess what? The medium-chain ones, often found in coconut oil and palm kernel oil (though palm oil is a whole other can of worms, ethically and environmentally speaking, but let's stick to the fat science for now!), might actually behave differently in our bodies. They're absorbed more directly and used for energy. It's like they get VIP access to the energy factory, skipping the usual baggage claim of LDL cholesterol!
Let's Look at Some Potential Statements and See If They're True or False
Okay, imagine you're faced with a multiple-choice question. Let's brainstorm some possibilities and see which ones might be the real deal. This is where we get to play detective!
Statement Idea 1: "Saturated fats are always bad for your health."
False! As we just talked about, this is a bit of an oversimplification. While excessive intake of certain saturated fats can be detrimental, it's not a black-and-white situation. Moderation is key, and the source of the saturated fat matters. For example, saturated fat from whole foods like dairy (full-fat yogurt, cheese in moderation) might be part of a balanced diet for some people, whereas the saturated fat from a highly processed donut is probably a different story. Our bodies are amazing, complex machines, and they don't always react to nutrients in a simple, one-size-fits-all way.
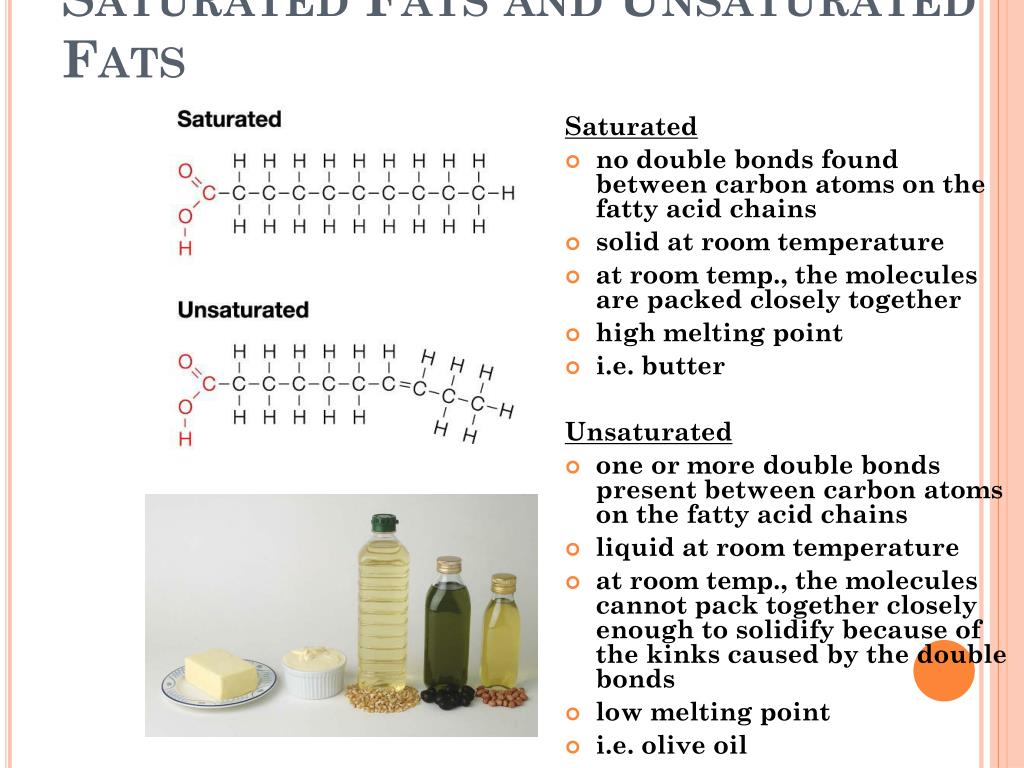
Statement Idea 2: "Saturated fats are liquid at room temperature."
False! This is a classic characteristic that helps us identify them. Remember our tightly packed suitcase analogy? That stability makes them solid at room temperature. Think of butter, lard, or coconut oil (which is solid below about 76°F or 24°C). Unsaturated fats, on the other hand, are the ones that tend to be liquid at room temperature – think olive oil or canola oil. They have kinks in their carbon chains, making them less able to pack together tightly.
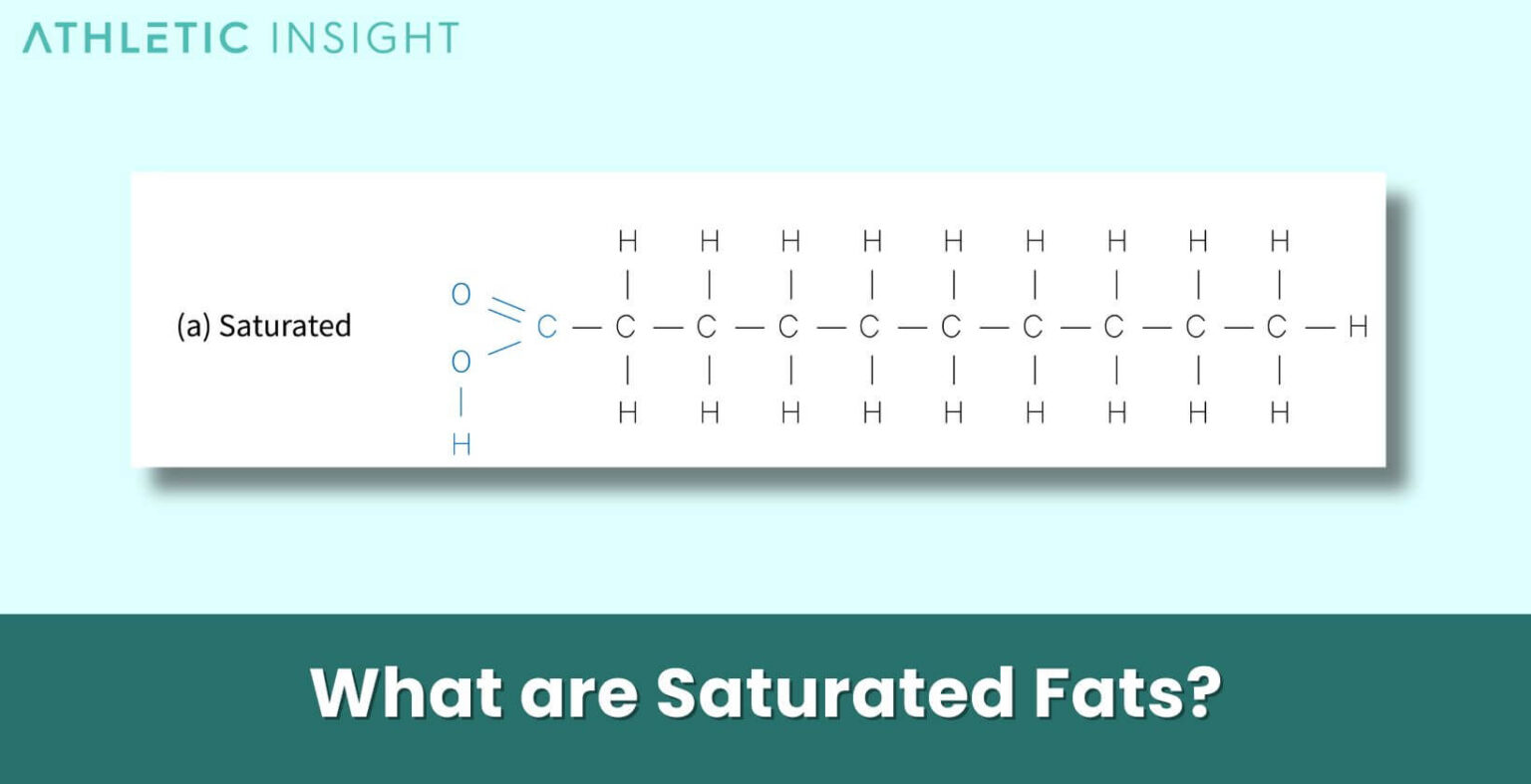
Statement Idea 3: "Saturated fats contain only single bonds between carbon atoms."
True! Ding, ding, ding! This is a fundamental chemical characteristic of saturated fats. Because they are "saturated" with hydrogen, each carbon atom can only form single bonds with its neighbors and with hydrogen. If there were any double or triple bonds between carbon atoms, it would mean fewer hydrogen atoms could be attached, and the fat wouldn't be saturated. It's like a full parking lot – no room for extra cars (hydrogen atoms)! This is a crucial point in their chemical structure.
Statement Idea 4: "All sources of saturated fat are equally unhealthy."
False! Again, this is too simplistic. While the amount of saturated fat is a consideration, the overall nutritional profile of the food matters. A piece of dark chocolate (which contains some saturated fat) might offer antioxidants, while a deep-fried candy bar likely offers a whole lot of sugar and very little else beneficial. Plus, as mentioned, the type of saturated fatty acid can vary. It's about the whole package, not just one ingredient!
Statement Idea 5: "Saturated fats are essential for certain bodily functions."
True! This is another important true statement! While we often focus on the potential downsides, fats, including some saturated fats, play vital roles in our bodies. They are crucial for absorbing fat-soluble vitamins (A, D, E, and K). They are building blocks for cell membranes, helping to keep our cells strong and functional. They also help insulate our organs and provide energy. So, while we don't want to overdo it, a certain amount is definitely necessary for us to thrive. It’s like having just enough insulation in your house to stay cozy, but not so much that you overheat!
Statement Idea 6: "Foods high in saturated fat are always processed foods."
False! Absolutely false! Many natural, whole foods are naturally high in saturated fat. Think of red meat, butter, cheese, and coconut. These are not processed foods in the same way a bag of chips or a frozen pizza is. The processing of food often adds unhealthy fats, but the presence of saturated fat doesn't automatically mean something is heavily processed. Nature gives us saturated fats too!
So, Which Statements are Generally True?
Based on our chat, the statements that are generally and scientifically true concerning saturated fats are:
- Saturated fats contain only single bonds between carbon atoms. (This is a fundamental chemical definition.)
- Saturated fats are essential for certain bodily functions. (They are necessary for vitamin absorption, cell structure, and energy.)
Remember, science is always evolving, and our understanding of nutrition is constantly being refined. What was considered gospel yesterday might be re-examined today. The key takeaway is to approach nutrition with a curious and open mind, focusing on a balanced diet with a variety of nutrient-rich foods.

It's not about demonizing entire food groups, but rather understanding the nuances. Think of it like building a really awesome playlist. You need a mix of tempos and styles to make it truly great. Your diet is similar!
So, next time you're pondering the fats in your food, remember the tightly packed suitcase, the single bonds, and the essential roles they play. And most importantly, remember that a little bit of knowledge goes a long way in making healthy choices that feel good and fuel your awesome life.
Keep exploring, keep learning, and keep enjoying delicious, nourishing food. Your body will thank you for it, and you'll be rocking that vibrant health with a smile! Now go forth and conquer your culinary adventures!
