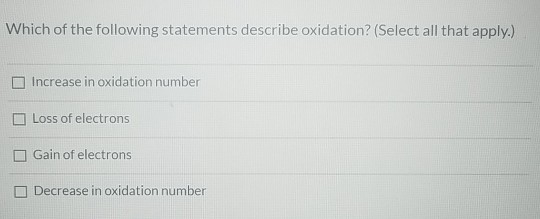
Which Of The Following Statements Are Associated With Oxidation

Hey there, science explorer! Ever feel like chemistry is just a bunch of complicated words and reactions that make your brain feel like it’s doing a super intense workout? Yeah, I get it. But today, we’re going to tackle a concept that’s actually pretty cool and, dare I say, fun. We're diving into the wild world of oxidation!
Think of oxidation like a little chemical drama playing out. It’s not as scary as it sounds, I promise. We’re going to chat about some statements and figure out which ones are like the main characters in the oxidation story. So, grab your imaginary lab coat (or just a comfy blanket, no judgment!), and let’s get this party started!
Oxidation: It's Not Just About Rusty Spoons!
First off, let's clear up a common misconception. When we hear "oxidation," our minds often jump straight to that annoying rust that forms on our bikes or old garden tools. And yeah, rust is definitely a form of oxidation! But oxidation is so much more than just things getting all crumbly and reddish-brown. It's a fundamental chemical process happening all around us, all the time.
It's like the universe's way of saying, "Hey, let's shuffle some electrons around!" And who doesn't love a good electron shuffle, right? Okay, maybe that’s not the most exciting thing to say, but stick with me. It’s going to get more interesting, I swear!
The Usual Suspects: What Does Oxidation Actually Mean?
So, what are the tell-tale signs of oxidation? What makes a statement ring true when it comes to this chemical phenomenon? Let's break down some of the key players. We're going to look at a few common scenarios, and you'll be a pro at spotting oxidation in no time. It’s like a chemistry scavenger hunt, but with less dirt and more molecules!
Statement 1: Loss of Electrons
Alright, here’s the big one, the headline news of oxidation: when a substance undergoes oxidation, it loses electrons. Think of it like a little electron giveaway. An atom or molecule is happily holding onto its electrons, and then, poof, it decides to pass some of them on.
Imagine you have a bag of your favorite candies. Oxidation is like you giving some of those candies away to a friend. You've lost candies, right? Same idea with electrons. The substance that loses electrons is said to be oxidized.
This is probably the most important thing to remember about oxidation. It's the core definition. If you see a statement talking about a substance donating or losing electrons, you can bet your bottom dollar it's related to oxidation. It’s the MVP of oxidation statements!

Statement 2: Increase in Oxidation State
Now, this might sound a little more technical, but it’s actually a super helpful way to track what’s going on. Each atom in a compound is assigned an "oxidation state." Think of it as a numerical score or a label that tells us how "electron-rich" or "electron-poor" an atom is.
When a substance is oxidized, its oxidation state increases. Why? Because it's losing negatively charged electrons! Losing negative charge means the overall charge or "state" becomes more positive (or less negative). It's like going from a score of -1 to +1. You've definitely gone up!
So, if you see a statement mentioning an increase in oxidation state, that’s a big, flashing neon sign pointing towards oxidation. It’s like the umpire calling "out!" – you know something has happened. This is a super handy trick for identifying oxidation, especially in more complex reactions.
Statement 3: Gain of Oxygen
Okay, remember how we talked about rust? That’s iron reacting with oxygen from the air. This is where the name "oxidation" really shines through! A classic sign of oxidation is the gain of oxygen.
When a substance reacts with oxygen and incorporates it into its structure, it's often undergoing oxidation. Think of it like oxygen being a very enthusiastic new team member that loves to join things. It binds to other elements and, in doing so, often leads to them being oxidized.
For example, when carbon burns in air, it forms carbon dioxide. Carbon atoms gain oxygen atoms, and this is a very clear example of oxidation. So, if a statement talks about a substance getting "oxygenated" or combining with oxygen, that's a strong contender for an oxidation statement. Easy peasy, right?
Statement 4: Loss of Hydrogen
Now, this one might seem a little less obvious at first, but it’s just as important! In many organic (carbon-based) reactions, oxidation is also associated with the loss of hydrogen atoms.
Think about it this way: in many organic molecules, hydrogen atoms are often bonded to carbon. When an oxidation reaction happens, those hydrogen atoms can be removed. It’s like a molecule shedding some of its hydrogen accessories.
For instance, an alcohol (like ethanol, the stuff in drinks, but in a chemical context!) can be oxidized to an aldehyde, and then further to a carboxylic acid. In these steps, hydrogen atoms are lost. So, if you see a statement mentioning the removal or loss of hydrogen from a molecule, particularly an organic one, it's a pretty good bet it's linked to oxidation.
Let's Put on Our Detective Hats!
So, we've got our main clues: loss of electrons, increase in oxidation state, gain of oxygen, and loss of hydrogen. Now, let’s imagine we’re given a list of statements, and we have to pick out the ones that scream "oxidation!" It's like a chemistry pop quiz, but without the pressure of a grade. More like a fun mental puzzle!
Statement A: A substance gains electrons.
Hmm, gains electrons? Wait a minute, we just said oxidation is about losing electrons! So, if a substance gains electrons, it's actually doing the opposite of being oxidized. This is called reduction. So, Statement A is a big fat NO for oxidation. Don't fall for that trick!
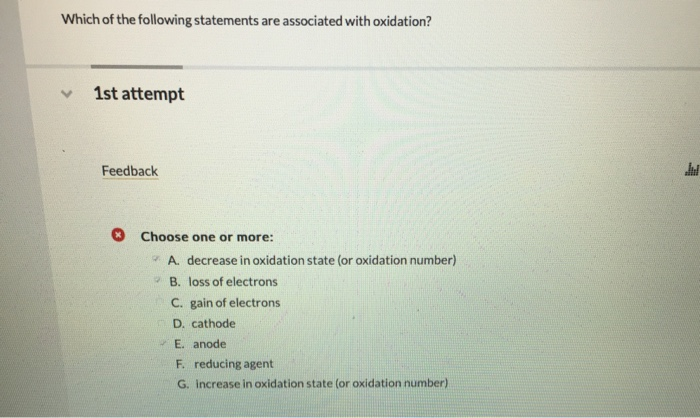
Statement B: A substance loses electrons.
Aha! This is our main man, our headline news! When a substance loses electrons, it is indeed being oxidized. So, Statement B is a definite YES! High five!
Statement C: A substance gains oxygen.
Remember our rusty spoons and burning carbon? Yes, the gain of oxygen is a classic indicator of oxidation. So, Statement C gets a big thumbs up!
Statement D: A substance loses oxygen.
Losing oxygen? That sounds like the opposite of what we just discussed for oxidation. In fact, losing oxygen is often associated with reduction. So, Statement D is another NO for oxidation. Keep those electrons and oxygen atoms straight!
Statement E: A substance's oxidation state increases.
We talked about oxidation state as a score. If the score increases, it means the substance has become less negative (or more positive) due to losing electrons. So, Statement E is a definite YES! It's a direct consequence of losing those negative little guys!
Statement F: A substance loses hydrogen.
For our organic friends, the loss of hydrogen is a tell-tale sign of oxidation. Think of those alcohols turning into aldehydes. So, Statement F is a YES!
Statement G: A substance gains hydrogen.
Gaining hydrogen? That's usually the opposite of oxidation in organic chemistry. This is more often associated with reduction. So, Statement G is a NO for oxidation.
The Final Verdict: Your Oxidation Superstars!
Drumroll, please! Based on our detective work, the statements associated with oxidation are:
- Statement B: A substance loses electrons. (This is the fundamental definition!)
- Statement C: A substance gains oxygen. (The classic, visually obvious one!)
- Statement E: A substance's oxidation state increases. (The numerical detective!)
- Statement F: A substance loses hydrogen. (Especially in organic chemistry!)
See? It’s not so scary after all! Oxidation is all about a substance becoming "electron-deficient" in one way or another, whether it’s by actually losing electrons, taking on oxygen (which is pretty electronegative and likes to pull electrons), or shedding hydrogen.
It’s like a chemical dance where electrons are passed around, and oxidation is just one of the dance moves! It’s a fundamental part of so many processes, from how our bodies get energy from food (cellular respiration is a whole lot of oxidation!) to how batteries work.
So, the next time you see something rust, or watch a fire burn, or even just think about your own biology, you can smile and know that a little bit of oxidation magic is happening. You’ve just unlocked a new level in your understanding of the world around you. Isn't that awesome? You’re basically a chemistry superhero now!
Keep exploring, keep questioning, and never let those chemical concepts dim your bright, curious mind. You’ve got this, and the world of science is so much brighter with you in it! Go forth and be scientifically awesome!
