Which Of The Following Statements About Subatomic Particles Is True

Hey there, awesome human! Ever look up at the stars and wonder what it's all about? Or maybe you've just finished a really good cup of coffee and feel this overwhelming sense of… well, everything? We're all connected, you know, in ways that are both mind-boggling and utterly delightful. And guess what? The secret sauce, the tiny, invisible building blocks that make it all happen, are these things called subatomic particles. Yep, we're talking about the really small stuff. Like, smaller than a microscopic dust bunny that’s hiding under your couch. We’re talking about the universe's tiniest LEGO bricks! Isn't that cool?
Now, let’s get real for a sec. The world of subatomic particles can sound super complicated, like something only folks in lab coats with wild hair would understand. But trust me, it’s also a place of incredible wonder and, dare I say, fun! It’s like unlocking a secret level in a video game, but the game is reality itself. And the best part? We’re going to tackle a little question that might seem a bit like a quiz, but it’s actually your gateway to understanding some seriously neat stuff. So, let’s dive in, shall we?
Here's the big question we’re pondering today: Which of the following statements about subatomic particles is true? Now, I’m not going to give you a pop quiz without some helpful hints. Think of this as a guided tour, not an exam. We’re going to explore some of the possibilities, and by the end, you’ll have a much clearer picture of why this whole subatomic world is so darn exciting.
Let's consider some of the usual suspects when we talk about subatomic particles. You've probably heard of electrons, right? They're like the zippy little workers that orbit the center of an atom, carrying a negative charge. They’re crucial for everything from the electricity that powers your phone to the way chemical bonds form. Without electrons, your coffee wouldn't be hot, and your favorite song wouldn't play. Pretty important little guys, wouldn't you say?
Then we have protons. These are found in the nucleus of an atom, smack-dab in the middle, and they carry a positive charge. Think of them as the steady anchors of the atom. Along with neutrons, they make up the bulk of the atom’s mass. They’re the reason one element is different from another. For example, the number of protons is what tells us if we're looking at a humble atom of oxygen or a fancy atom of gold. Wild, huh?
And let's not forget neutrons! Also hanging out in the nucleus, these guys are like the neutral mediators. They don’t have an electrical charge, but they contribute to the atom's weight and stability. They’re the silent backbone, keeping things together. Imagine them as the calm friends at a party, making sure everything doesn’t get too chaotic.
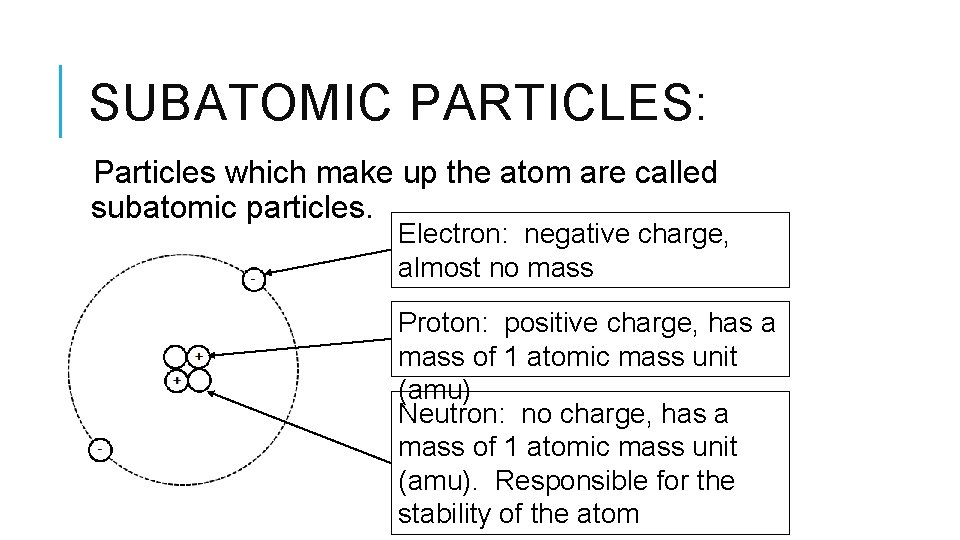
So, we've got electrons, protons, and neutrons. These are often called the "fundamental" particles of an atom. But here’s where things get even more interesting. The universe is a much grander and more intricate place than just those three. The reality is, our understanding of subatomic particles has evolved over time, and it's still a buzzing, vibrant field of research. Scientists are constantly discovering new particles and uncovering deeper layers of reality. It's like an ongoing mystery novel where the plot twists are truly out of this world!
The Quirks of the Quantum Realm
Now, if we were to say something like, "All subatomic particles are big and heavy," that would be a pretty obvious nope, right? I mean, they're subatomic – that implies they’re incredibly small! So, statements that contradict this fundamental characteristic are usually the ones to watch out for. Size matters, even when we're talking about things you can't see without some seriously fancy equipment.
What about their behavior? This is where the fun really kicks in. Subatomic particles don't always play by the rules we're used to in our everyday lives. For instance, the idea that a subatomic particle can be in multiple places at once? Sounds like science fiction, but in the quantum realm, it’s a thing! This is called superposition, and it's one of the most mind-bending concepts in quantum mechanics. It’s like your keys being both on the table and in your pocket until you actually look for them. Okay, maybe not exactly, but you get the idea – reality gets a little fuzzy down there!

And then there's entanglement. This is when two or more particles become linked in such a way that they share the same fate, no matter how far apart they are. It's like having a cosmic connection that transcends space and time. If you do something to one entangled particle, the other one instantly reacts. Einstein famously called this "spooky action at a distance." Spooky? Maybe. But also incredibly cool and a testament to the interconnectedness of everything.
So, if one of our statements declared something like, "Subatomic particles always behave in predictable, classical ways, just like a billiard ball," we'd have to raise an eyebrow. While protons and neutrons are pretty stable, electrons, for example, are often described as being in a "probability cloud" around the nucleus, rather than having a fixed orbit. It’s less like a planet orbiting a star and more like a fuzzy dance of possibilities.
Beyond the Basics: A Particle Zoo!
But wait, there's more! Our universe is a vibrant and bustling particle zoo. Beyond electrons, protons, and neutrons, there are a whole host of other subatomic particles that scientists have discovered. We’re talking about things like quarks, which are the building blocks of protons and neutrons themselves. And photons, the particles of light! Every ray of sunshine, every flash of your camera, is made of these little packets of energy. Pretty neat, right? Imagine holding a tiny beam of light in your hand – that’s kind of what a photon is!

There are also neutrinos, which are incredibly elusive particles that zip through matter almost without interacting. They’re like cosmic ghosts, passing through us and the entire Earth by the billions every second! We can't feel them, we can't see them, but they are there, constantly on their journey. It’s a humbling thought, isn’t it? That we’re constantly being permeated by these invisible travelers.
So, if a statement claimed something like, "Protons and neutrons are the only fundamental particles in an atom," that would also be a bit off the mark. They are fundamental to atoms, yes, but the deeper we look, the more we find. Our understanding of the fundamental forces of nature is also tied to specific particles, like the gluons that hold quarks together and the W and Z bosons involved in certain types of radioactive decay.
Putting It All Together
Let’s circle back to our initial prompt: Which of the following statements about subatomic particles is true? Without seeing the exact statements, we can infer some key principles that often hold true in this fascinating realm:

- Subatomic particles are incredibly small. This is a foundational truth. Anything claiming they are large or macroscopic is likely incorrect.
- Subatomic particles exhibit quantum behavior. This means they can behave in ways that seem counterintuitive to our everyday experience, like superposition and entanglement. Statements that deny this quantum weirdness are usually false.
- The universe is made up of a variety of subatomic particles. It's not just electrons, protons, and neutrons. There's a whole zoo of them out there, each playing a vital role. A statement that limits the known subatomic particles to just a couple would be incomplete, and therefore, potentially misleading.
- These particles are fundamental to the structure of matter and the forces of nature. They are the universe's ultimate building blocks and the drivers of all interactions.
So, when you’re faced with options, look for the one that acknowledges the tiny nature of these particles, their unique quantum properties, and the rich diversity of the subatomic world. These are the hallmarks of true statements about subatomic particles.
Isn't this just amazing? The fact that we, as conscious beings, can even begin to comprehend these tiny, invisible entities that make up everything we see, touch, and experience, is a testament to the incredible power of human curiosity. It’s a reminder that even when things seem overwhelmingly complex, there’s always beauty and wonder to be found in exploring the unknown.
So, the next time you’re contemplating the vastness of the universe, remember the minuscule but mighty subatomic particles that are its foundation. They are the silent architects of reality, the unheralded heroes of existence. And the more you learn about them, the more you’ll realize that the universe is not just a place to be observed, but a magnificent, intricate, and utterly awe-inspiring playground waiting for you to explore. Go forth and be curious!
