Which Of The Following Statements About Polar Molecules Are True

Hey there, science explorers! Ever wondered about those teeny-tiny building blocks of everything around us? Well, get ready to dive into the absolutely fascinating world of polar molecules. They're not just boring science terms; they're actually the lifeblood of so many cool things we see and experience every single day!
Imagine a little party happening at the molecular level. Some molecules are perfectly balanced, like a perfectly symmetrical dance. Everyone's sharing the spotlight equally. But then you have these other guys, the polar molecules, who are a bit more… dramatic!
Think of it like a friendly tug-of-war. In a polar molecule, some parts are a little more "popular" with electrons than others. This means there's an uneven distribution, like one end of the rope being pulled a bit harder. It creates a tiny, but mighty, electrical difference.
So, what makes these guys so special? Well, this uneven pull is what gives them their unique personalities and makes them do all sorts of amazing tricks. It’s like having molecules with a built-in plus and minus sign!
Let's spill the tea on some of the awesome things that are true about these captivating characters. Get ready for some mind-blowing revelations that might just change how you look at the world!
The Magnetic Attraction
One of the most captivating things about polar molecules is their ability to attract each other. It’s like they have a built-in charm offensive!
Remember that friendly tug-of-war we talked about? Because one end is a bit more positive and the other a bit more negative, opposite ends just naturally want to get close. It's the ultimate cosmic matchmaking!
This attraction is why things like water, a super common polar molecule, can stick together so well. It's not magic; it's just good old-fashioned molecular chemistry at play. Pretty neat, right?

The Universal Solvent Superstars
Speaking of water, polar molecules are often hailed as the universal solvents. And let me tell you, they totally earn that title!
Because they have those charged ends, polar molecules are brilliant at dissolving other polar substances. They’re like tiny molecular huggers, surrounding and pulling apart other charged particles.
This is why sugar dissolves in your tea or salt disappears in water. The water molecules, being polar, are just so good at interacting with and pulling apart the sugar and salt molecules. It’s a party where everyone gets invited… and dissolved!
But here’s a fun twist: nonpolar molecules, on the other hand, are usually not good friends with polar solvents. It’s like oil and water – they just don’t mix because they have completely different personalities.
The High Boiling Point Bonanza
Another cool trait of polar molecules? They often have surprisingly high boiling points. You'd think they'd be eager to escape, but nope!

This is all thanks to those intermolecular forces. The strong attractions between polar molecules require a lot of energy to break apart. That means more heat is needed to turn them into a gas.
Think about water again. It's a liquid at room temperature, but it takes a good amount of heat to make it boil and turn into steam. That’s the power of polar attraction at work, keeping those molecules cozy!
The Electrical Conductivity Charm
While not all polar molecules are great electrical conductors on their own, they play a crucial role in making certain things conduct electricity.
When polar molecules like water dissolve ionic compounds (think salts), they can help separate the charged ions. These free-moving ions are what carry the electrical current.
So, while the water itself might not be zapping, its ability to dissolve and disassociate other charged particles is key to conductivity in many solutions. It’s like they’re setting the stage for the electrical show!
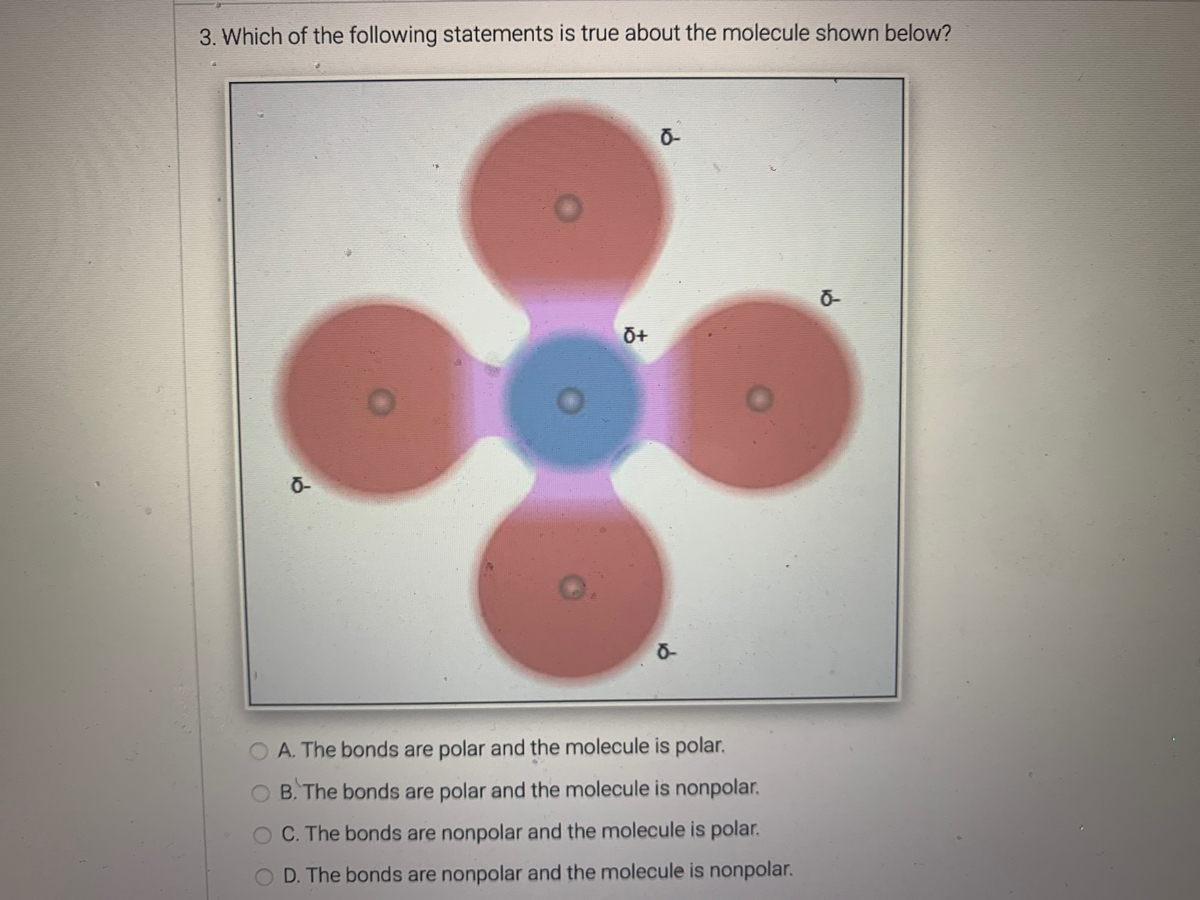
The Shape Matters!
It's not just about which atoms are bonded together; the shape of a molecule is super important in determining if it's polar. Even if you have polar bonds, if the molecule is symmetrical enough, the charges can cancel out!
Imagine a perfectly balanced scale. Even if you put weights on each side, if they are perfectly balanced, the scale stays level. It’s a similar idea with molecular symmetry.
This is why carbon dioxide (CO2) is nonpolar. It has polar bonds between carbon and oxygen, but the molecule is linear and symmetrical, so the pulls cancel out. Water (H2O), on the other hand, has a bent shape, which means the pulls don’t cancel, making it wonderfully polar!
The Biological Ballerinas
Now, for the really mind-blowing part: polar molecules are the absolute superstars of biology! Life as we know it wouldn't be possible without them.
Water, our favorite polar molecule, is essential for all known life. It’s the medium where most biological reactions happen. It transports nutrients, regulates temperature, and so much more!

Proteins, DNA, and many other vital biological molecules are also polar or have polar regions. This polarity is what allows them to interact with each other, fold into their complex shapes, and carry out their incredible functions.
Without these charged interactions, your cells wouldn’t be able to communicate, your muscles wouldn’t work, and your brain wouldn’t even be able to think! It’s a testament to the power of these seemingly simple molecular traits.
The Everyday Enchantment
So, the next time you’re sipping a drink, washing your hands, or even just breathing, take a moment to appreciate the unsung heroes: polar molecules.
They are the reason your soap works, your food dissolves, and your body functions. They are the invisible architects of so many of the conveniences and wonders of our daily lives.
It’s a little bit like discovering a secret superpower that these tiny particles possess. They’re not just atoms and bonds; they’re the reason for so much of the magic we experience, from the grand to the mundane. Go ahead, be amazed!
