Which Of The Following Statements About Dehydration Reactions Is False

Hey there, fellow humans! Let’s talk about something we’ve all probably experienced, even if we didn’t call it by its fancy scientific name. We’re diving into the wild, weird world of dehydration reactions. Now, don’t let the “reaction” part scare you. It’s not like a toddler having a meltdown because you ate the last cookie (though, sometimes, that can feel like a chemical reaction, right?).
Think about it. Remember that time you were absolutely parched, like a desert explorer who’d forgotten their canteen for three days? Your mouth felt like sandpaper, your tongue was sticking to the roof of your mouth like a confused gecko, and your brain felt like it was running on low battery. That, my friends, is your body screaming “DEHYDRATION!” It’s basically your body throwing a tantrum because it’s running out of its essential liquid fuel.
So, what’s the big deal with dehydration? Well, our bodies are like tiny, incredibly complex water parks. Water is the main attraction, the coolant, the lubricant, and the delivery service. It carries nutrients, gets rid of waste, and keeps everything running smoothly. When you’re dehydrated, it’s like the water slides have been shut down, the lazy river is empty, and the lifeguards are all taking a nap. Not a good scene.
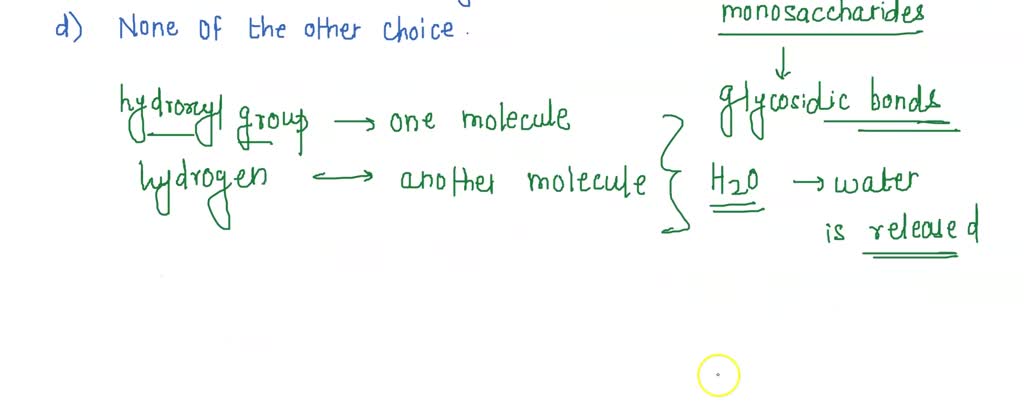
Now, when scientists talk about “dehydration reactions” in chemistry, they’re talking about a specific kind of event. It’s not just about feeling thirsty. It's about molecules doing a little dance, and in this dance, a molecule of water (H₂O) gets kicked out. Imagine two buddies, maybe a really big molecule and a slightly smaller one, who are hanging out. To join forces and become an even bigger, more awesome molecule, they decide to shed a water molecule. It’s like they’re saying, “Okay, to become one super-molecule, we gotta ditch this little extra bit. Water, you’re up!”
It’s kind of like when you’re packing for a trip and you’ve got way too much stuff. You look at your suitcase, you look at that one extra sweater, and you think, “Do I really need this?” So, you take it out. That sweater is like the water molecule. It’s being removed so that the remaining things can fit together better, or in the case of molecules, form a new, often more stable, compound. Pretty neat, huh?
These dehydration reactions are super important in the grand scheme of things. They’re the building blocks for so many things in our bodies. Think about how your muscles are made, or how your DNA is structured. A lot of that involves molecules linking up, and sometimes, that linkage happens because a little water molecule is saying, “See ya!”
Let’s get a bit more specific, but don't worry, we're not going to pull out the advanced calculus here. We're keeping it casual. A classic example is when sugars link up to form bigger carbohydrates. Imagine LEGO bricks. Individual LEGO bricks are like simple sugars. When you click them together to build a spaceship or a castle, that’s kind of like a dehydration reaction. You take two little sugar molecules, they link up, and poof, a water molecule is released. Now you have a bigger sugar molecule, a disaccharide, like sucrose (table sugar) or lactose (milk sugar).
It's like when you’re making a really long chain of paper clips. Each time you connect two paper clips, you’re essentially forming a bond, and sometimes, the process of forming that bond requires a little bit of… let’s call it “energetic shedding.” In the case of dehydration reactions, that shedding is a water molecule.
Another place we see this is when we’re building proteins. Proteins are the workhorses of our cells. They do pretty much everything. They are made up of smaller units called amino acids. When amino acids link up to form a long protein chain, it's a series of dehydration reactions. Each time an amino acid joins the chain, a water molecule is removed. It’s like a molecular construction site where the workers are linking up building blocks, and the excess packaging (the water molecule) is neatly disposed of.
So, to recap the vibe: dehydration reactions involve molecules joining together by releasing a water molecule. It's a constructive process, not a destructive one in the sense of breaking things down. It's about making something bigger or more complex by shedding something smaller.
Now, Let’s Talk About the False Statement
Here’s where we put on our detective hats. We’re going to look at some statements about dehydration reactions, and one of them is going to be a total fib. It’s going to be like finding a unicorn at a dog show – unexpected and definitely out of place.
Let’s imagine some statements. We’ll make them sound plausible, like that friend who tells you they saw a UFO last week. You want to believe them, but… maybe not entirely.
Statement Option A: “Dehydration reactions are crucial for forming complex carbohydrates from simple sugars.”
Does this sound right? We just talked about sugars linking up, right? Like those LEGO bricks. So, when simple sugars (monosaccharides) join to form bigger sugars (disaccharides, polysaccharides), a water molecule is indeed released. Think of starch in potatoes or cellulose in plants – those are big, complex carbohydrates built this way. This statement sounds pretty darn true. It’s like saying “water is wet.” Yep, checks out.
Statement Option B: “Dehydration reactions involve the addition of a water molecule to break a larger molecule into smaller ones.”
Whoa there, partner! Does this one sound a little… backward? Remember how we said dehydration reactions are about removing water to join things? This statement talks about adding water to break things. That sounds more like a hydrolysis reaction. Hydrolysis is literally “water splitting.” Think of trying to break apart a tightly packed suitcase. Sometimes, you need to add a bit of something (like a crowbar, or in chemistry, water) to pry things apart. So, this statement sounds like it’s describing the opposite of a dehydration reaction. This one’s giving us the shivers, and not in a good, cozy way.

Statement Option C: “Amino acids link together via dehydration reactions to form polypeptide chains, which are the building blocks of proteins.”
We covered this earlier, didn’t we? Those protein-building powerhouses! Amino acids don't just hang out; they get linked up in a specific order to make all the amazing proteins our bodies need. And how do they link up? By shedding a water molecule at each step. So, this statement is singing our tune. It’s true, just like that feeling of accomplishment after you’ve finally tidied up your room.
Statement Option D: “Dehydration reactions are fundamental processes in both the synthesis of macromolecules and in cellular respiration.”
Now, this one is a bit of a double-header. Dehydration reactions are definitely fundamental for building macromolecules (like carbohydrates, proteins, and nucleic acids – think DNA!). That part is solid. But cellular respiration? That’s the process where our cells break down glucose to get energy. It involves a lot of complex steps, and while there are hydrolysis reactions happening (breaking things down with water), the primary role of dehydration reactions isn’t in the energy-releasing steps of breaking down glucose. It’s more about building things up. So, while dehydration reactions are involved in many cellular processes, saying they are "fundamental" in cellular respiration in the same way they are in building macromolecules might be a slight stretch, or perhaps a misleading oversimplification. However, compared to Statement B, which is a direct contradiction, this one is more nuanced.
Let's think about it this way. Imagine you're building a house (macromolecule synthesis). You're constantly joining materials together, and sometimes, you have to get rid of excess bits (like sawdust). That’s dehydration. Now, imagine you’re demolishing an old house to build a new one (cellular respiration involves breaking down fuel). You’re definitely breaking things, and sometimes you use water to help break things down (hydrolysis). While there might be some very minor building-up steps occurring in complex pathways, the fundamental process of getting energy from fuel is about breaking bonds, not primarily forming them through dehydration.
However, if we're forced to pick the one statement that is unequivocally false, Statement B is the clear winner. It describes the exact opposite process. Statement D is more of a gray area, where the emphasis might be slightly off, but not entirely incorrect in the broader context of cellular metabolism.

So, let’s zoom in on Statement B again. It’s like saying, “To get a hug, you need to push someone away really hard.” That just doesn’t compute, does it? Dehydration reactions are about making things stick together by letting go of water. Adding water is for breaking things apart.
Think of it like this: when you’re making a delicious smoothie, you’re adding liquids (water, milk, juice) to break down the fruit and blend it all together. That's kind of like hydrolysis. But when you’re baking a cake, you’re mixing dry ingredients and wet ingredients, and the heat of the oven helps them come together, and sometimes during the chemical reactions within the batter, water molecules are released as things bond. It’s a subtle but important difference.
So, to sum up our investigation, if you see a statement that says dehydration reactions are about adding water to break things apart, you can confidently put your hand up and say, “Nope! That’s a big ol’ fib!” That, my friends, is the false statement about dehydration reactions. The other statements describe the real deal, the molecular magic that keeps our bodies and the world around us humming along.
Remember, the next time you feel thirsty, it's your body’s way of telling you it needs more of its essential fluid. And when you hear about dehydration reactions in science, think of molecules doing a little dance, shedding a water molecule, and becoming something bigger and better. It’s a beautiful, essential process, and now you know which statement is totally bogus!
