Which Of The Following Sn2 Reactions Is The Fastest
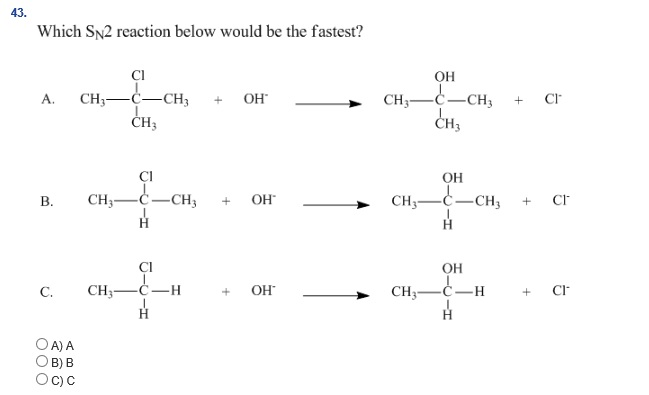
Alright, so picture this: you're chilling, maybe sipping on a latte, and someone casually drops the question, "Which of these SN2 reactions is the fastest?" Sounds kinda niche, right? But trust me, it’s actually a total blast to dive into. Think of it like a tiny, super-fast race happening at the molecular level. Who doesn't love a good race?
We're talking about
And here's the cool part: in an SN2 reaction, the nucleophile comes in from the back. It's like a sneaky ninja move. As it attacks, the leaving group peace out from the front. It’s a one-step deal. Bam! Done. No messing around. That's why it’s so zippy. And this back-attack thing? It’s super important. It flips the molecule inside out, like turning a glove inside out. Wild, right?
So, the big question is: what makes one of these little molecular dances faster than another? It’s all about making that sneaky ninja move as easy as possible. We’ve got a few key players that speed things up, and a few that make it a total crawl. It’s like picking the fastest race car – gotta have the right engine, the right tires, and a clear track!
The Speedy Contenders: What Makes It Zip?
First up, let’s talk about the substrate. This is the molecule doing all the heavy lifting (or, well, the letting go). Imagine trying to get through a crowded room. If you’re trying to reach someone, and there are tons of people blocking your path, it’s gonna be slow. Molecules are kinda the same.
The less crowded the carbon atom where the action is happening, the faster the SN2 reaction. We're talking about steric hindrance. Big, bulky groups attached to that carbon are like a bunch of sumo wrestlers in the way. They make it super hard for our nucleophile ninja to get in there and do its thing.
So, the fastest SN2 reactions happen on primary (R-CH2-X) and especially methyl (CH3-X) substrates. Think of a tiny little carbon atom with just a couple of hydrogens and the leaving group. So much open space! It’s like a deserted highway for our nucleophile. It can just zoom right in.
Then comes secondary (R2-CH-X) substrates. A little more crowded, a bit like a moderately busy street. Still doable, but not as lightning-fast as the primary guys.
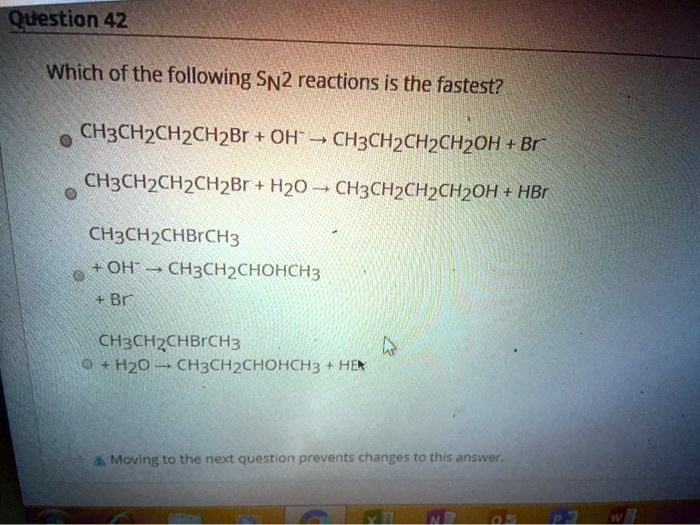
And then… the tertiary (R3-C-X) substrates. Oh boy. These are like a packed festival on a Saturday night. Those three bulky R groups are throwing a serious party, and our nucleophile is definitely not invited to attack. Tertiary substrates barely do SN2 reactions at all. They’re more into a different kind of molecular dance called SN1, which is a whole other story for another day. But the point is, for SN2, more clutter equals slower speed.
The Nucleophile: The Star of the Show!
Next up, we have the nucleophile. This is the molecule that’s doing the attacking. It’s the one with the goods – those precious electrons it’s offering up. And just like in real life, some attackers are way more enthusiastic (and effective!) than others.
What makes a nucleophile a speed demon? It’s gotta be strong. A strong nucleophile is one that’s really good at sharing its electrons. Think of it as someone who’s super generous with their snacks. They’re just eager to give them away!
Generally, charged nucleophiles are stronger than their neutral counterparts. For example, hydroxide (OH-) is way stronger than water (H2O). The negative charge makes it practically beg to share electrons. It’s like, "Take my electrons! Please! I have too many!"
Also, within a period on the periodic table, nucleophilicity increases as you go from right to left. So, something like a fluoride ion (F-) is actually a weaker nucleophile than, say, a bromide ion (Br-) in polar protic solvents, even though fluoride is more electronegative. This can seem a bit counterintuitive, but it’s all about how well the nucleophile can get at the substrate. Those bulky solvent molecules can get in the way of the smaller, more highly charged nucleophiles.

But here’s a quirky fact: in polar aprotic solvents (solvents that don’t have those messy H-bonds hanging around), the trend flips! Smaller, more concentrated nucleophiles like fluoride actually become stronger. It’s like all the competition is gone, and the little guys can finally shine! It’s a whole different party atmosphere.
So, a strong nucleophile is key. It's the eager beaver of the reaction world, ready to jump in and make things happen. A weak nucleophile? They’re more like the folks who stand around at the party, politely declining offers.
Leaving Groups: The Ones Who Say Goodbye
And finally, we have the leaving group. This is the part of the substrate that decides to pack its bags and leave. For an SN2 reaction to be fast, the leaving group needs to be, well, a good leaver. It needs to be stable on its own once it’s detached.
Think of it like a friend leaving a party. If they leave and immediately get into a huge fight, they’re probably not going to want to leave again anytime soon. But if they leave and go hang out with other cool people and have a great time, they’re happy to go!
Generally, the weaker the base, the better the leaving group. This is because weaker bases are more stable on their own. They’re less likely to want to go back and re-form that bond.
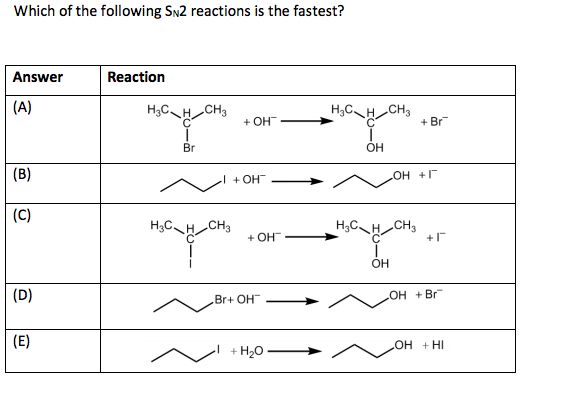
So, halide ions are pretty good leaving groups. Iodide (I-) is excellent. Bromide (Br-) is good. Chloride (Cl-) is okay. Fluoride (F-)? Not so great. It’s a pretty strong base, so it’s not super happy to be on its own.
Other good leaving groups include things like tosylates (-OTs) and mesylates (-OMs). These guys are like the rockstars of leaving groups. They’re super stable and always ready for their solo performance.
A bad leaving group would be something like a hydroxide ion (OH-). It's a strong base and would rather stick around than go off on its own. If you have a hydroxide attached, you usually need to do something to it first, like protonate it (add an H+) to make it a better leaving group, like water (H2O).
Putting It All Together: The Fastest Combination
So, if we were to have a lineup of potential SN2 reactions and you had to pick the absolute quickest, you'd be looking for a perfect storm of speed factors.
You want a methyl or primary substrate. Lots of open space! No sumo wrestlers allowed!

You want a strong nucleophile. That eager beaver ready to share electrons!
And you want a great leaving group. The one who’s ready for their independent career!
For example, the reaction of iodide (I-) with methyl iodide (CH3I) is pretty darn fast. Or even better, iodide with something like methyl tosylate (CH3OTs). That tosylate is like the ultimate VIP pass for leaving!
On the flip side, a tertiary substrate with a weak nucleophile and a poor leaving group would be agonizingly slow. It would be like watching paint dry… in slow motion… on a rainy day.
It's these little molecular dances, these energetic races, that make organic chemistry so fascinating. It's all about understanding the personalities of these molecules and how they interact. So next time someone asks about SN2 reactions, you'll know it's not just some boring science thing. It's a speed challenge, a popularity contest, and a farewell tour, all rolled into one! Pretty neat, huh?
