Which Of The Following Represents An Acid Base Neutralization Reaction

Hey there, science curious folks! Ever wondered what happens when you mix certain things together? Like, really mix them? Well, get ready to have your mind tickled, because we're diving into something super cool called an acid-base neutralization reaction. Sounds fancy, right? But trust me, it's more like a delightful dance party for molecules. And what's not to love about a good dance party?
Think of it like this: some things in the world are a bit ... well, spicy. We call these acids. They're the ones that can make your tongue tingle (if you're brave enough to taste them, which, by the way, you probably shouldn't do without expert supervision!). Think of things like the tangy stuff in your lemonade, or the fizz in your soda. They've got a certain zest, a bit of a bite.
Then, on the other side of the aisle, we have the bases. These guys are the opposite. They're often a bit slippery, and they can feel a bit soapy. Think of cleaning products, or even some stuff that helps you digest your dinner. They're the mellow, smooth operators of the chemical world.
Now, imagine our spicy acid and our mellow base meeting. It's like they've been looking for each other! They come together, and poof! They neutralize each other. It's not a fight; it's more like a big, warm hug. They combine in a way that makes both of them less ... themselves. The acidity goes away, and the basicness disappears. It's like they've found perfect balance.
And what's left after this amazing chemical embrace? Well, that's the really neat part. Usually, you end up with water and a salt. Yep, just like the stuff you put on your fries! It’s like they’ve retired from their intense acid/base careers and decided to settle down into a nice, calm life as H2O and some yummy (or not so yummy, depending on the salt!) mineral. How's that for a happy ending?

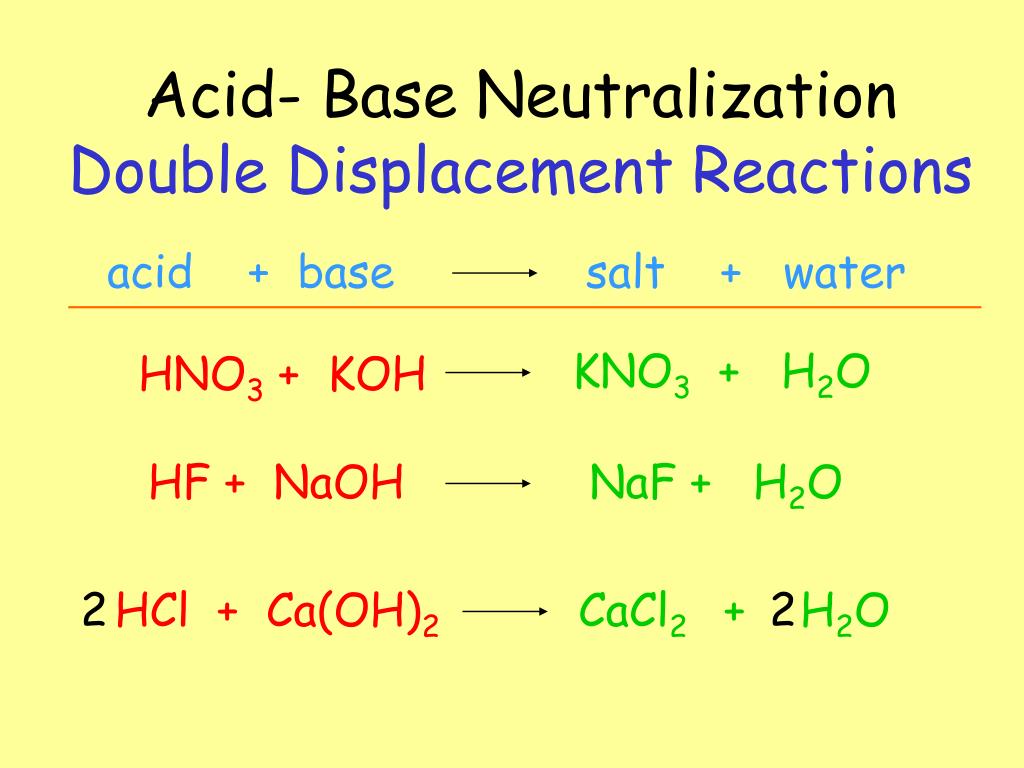
So, when you see a question asking which of the following represents an acid-base neutralization reaction, you're looking for that magical meeting. You're looking for an acid (think of the name starting with "hydro-" or ending in "-ic acid" or containing "sulfate", "nitrate", "phosphate", "carbonate", or other polyatomic ions associated with acids) bumping into a base (often something ending in "-oxide", "-hydroxide", or "-amine").
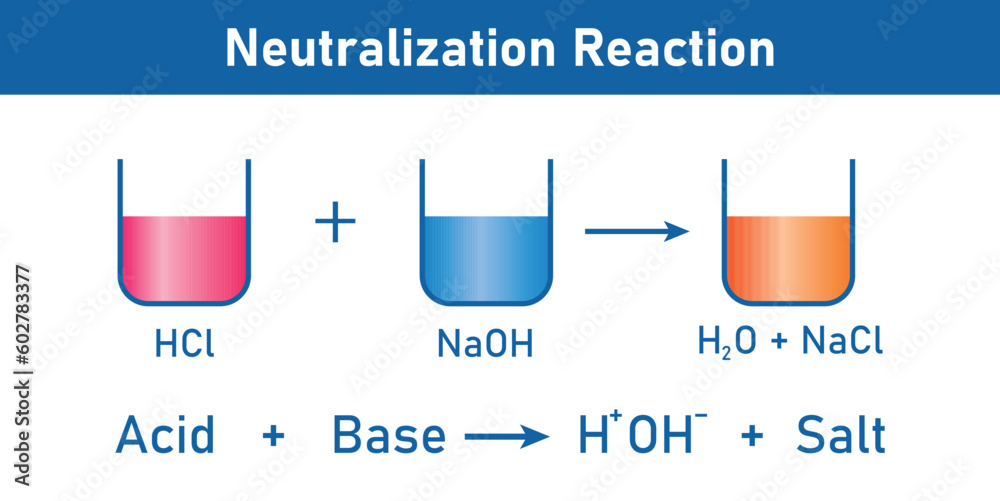
Let's consider a classic example. Imagine we have hydrochloric acid. That's a classic acid. It's pretty strong. And then we have sodium hydroxide. That's a classic base. When these two meet, it's like a celebrity couple hitting the red carpet. They're making a big statement!
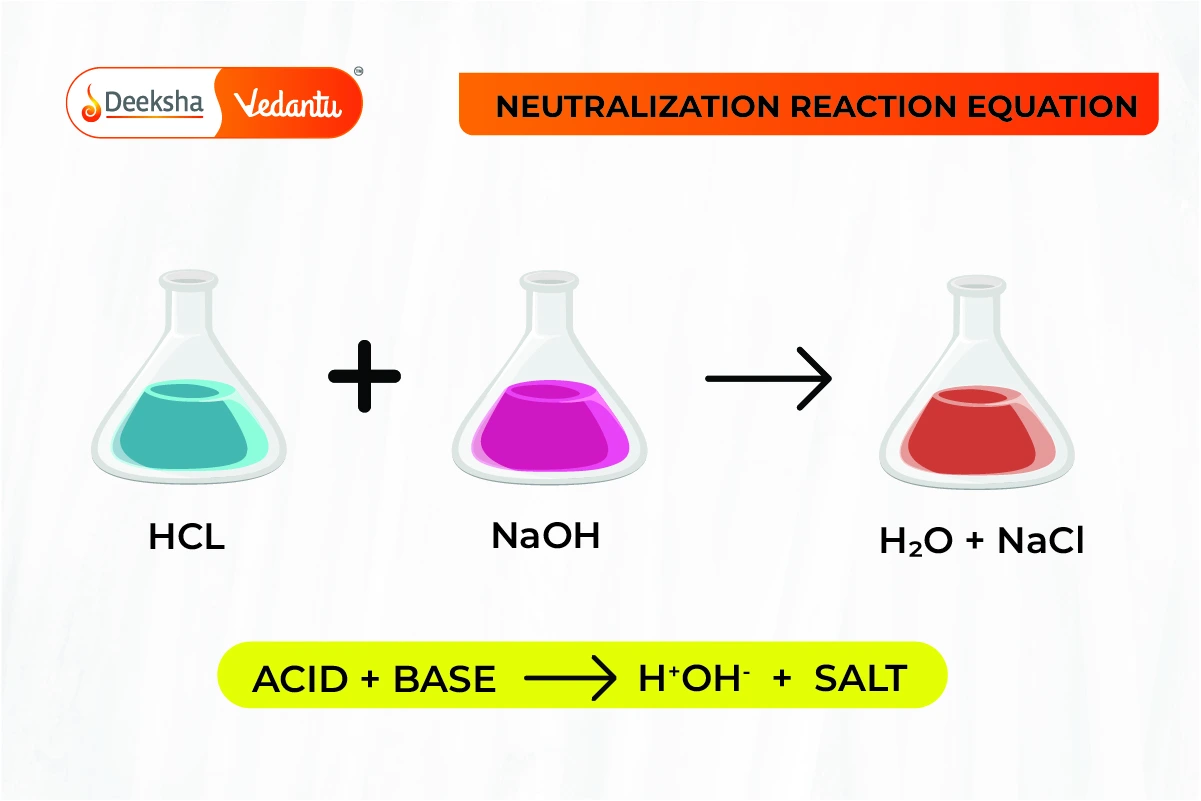
Hydrochloric Acid + Sodium Hydroxide → Sodium Chloride + Water
See? The hydrochloric acid (the spicy one) and the sodium hydroxide (the slippery one) come together. And what do we get? Sodium chloride (hello, table salt!) and water (essential for life, and totally neutral!). It's the ultimate transformation. The dramatic zing of the acid and the smooth feel of the base both vanish, leaving behind something completely new and, in this case, quite useful.
It's this very transformation that makes these reactions so fascinating. It's not just a boring chemical process; it's a fundamental concept that explains so many things around us. Think about antacids! When you get heartburn, that burning sensation is too much acid in your stomach. You take an antacid, which is a base, and voila! The base neutralizes the excess acid, and you feel relief. It's a mini neutralization reaction happening right inside you!
Or consider how farmers adjust the pH of their soil. If soil is too acidic, they add a base (like lime) to bring it back to a happy, neutral state for plants to grow. It’s like giving your garden a spa treatment! These reactions are the unsung heroes of our everyday lives, quietly working to keep things balanced and comfortable.
So, when you're faced with a chemical equation or a description of a mixture, keep an eye out for this special kind of interaction. You're looking for the signs of that dramatic meeting: an acid, known for its zest, encountering a base, known for its smooth demeanor. And the result? A calm, stable partnership, often producing the simple, essential elements of water and salt.
It's a reminder that sometimes, the most intense things can lead to the most peaceful outcomes. It’s the chemistry of balance, the art of settling differences, and the magic of transformation all rolled into one. Isn't that just the coolest? It makes you want to peek behind the curtain of the chemical world and see all the other amazing reactions happening!
So, next time you hear about an acid-base neutralization, don't just think of a dull lab experiment. Think of a lively chemical duet, a delicious transformation, and a fundamental force that keeps our world in perfect harmony. It's a small reaction, but its impact is huge, and that's what makes it truly special. Keep exploring, keep wondering, and you'll find these amazing reactions everywhere!
