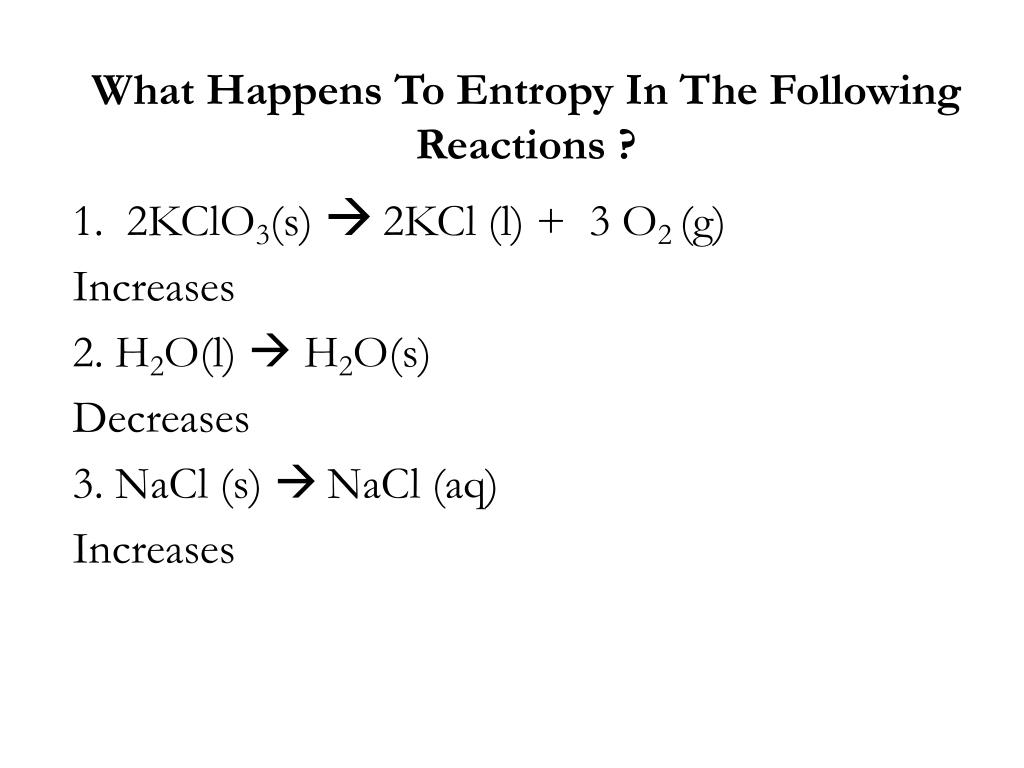
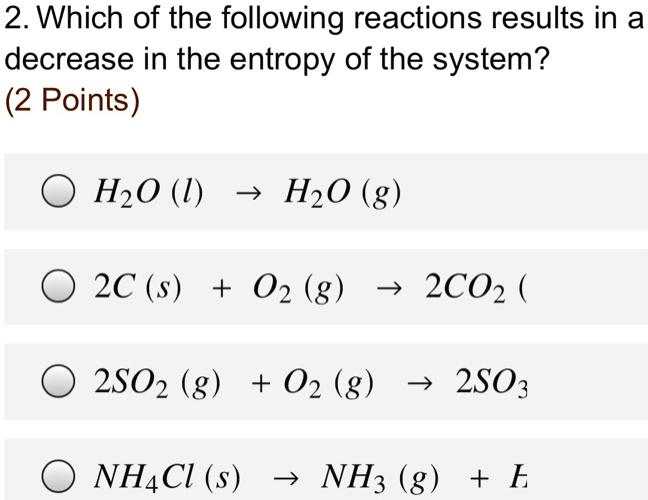
Which Of The Following Reactions Would Result In Decreased Entropy
Ever wondered if chemistry could be… fun? Like, really fun? Forget boring textbooks and confusing diagrams. We’re diving into a world where tiny particles do their own exciting dance, and we get to peek behind the curtain.
Today, we’re playing a little game. It’s a bit like a mystery, a puzzle wrapped in an enigma, but with atoms and molecules as the stars. We’re going to look at some chemical reactions, and our mission, should we choose to accept it, is to spot the one that’s a bit more… organized. A little less chaotic, if you will.
Think of entropy as a measure of messiness. The universe generally loves a good mess. Things tend to spread out, mix up, and generally get a bit more disordered. It’s the cosmic equivalent of your laundry pile spontaneously deciding to take over your bedroom.
So, when we’re looking for a reaction that results in decreased entropy, we’re actually looking for something that brings order to the chaos. It’s like tidying up that laundry pile, or maybe even folding it neatly! That’s the magic we're trying to find.
Let’s set the scene. Imagine a bustling party. Lots of people mingling, talking, moving around. That’s high entropy – lots of random movement and interaction. Now, imagine everyone suddenly sitting down at a long table, perfectly in their assigned seats, ready for a formal dinner. That’s decreased entropy. Things are much more structured, much less random.
Our chemical reactions are a lot like these parties. Sometimes they’re wild and chaotic, with molecules bouncing around like excited toddlers. Other times, they’re more subdued, more… intentional. And we’re on the hunt for those intentional ones.
We’ve got a few contenders, a few chemical scenarios playing out. Each one has its own unique personality and its own way of behaving. Some are explosive, some are quiet, and some… well, some are just trying to bring a little bit of order to the molecular world.
So, let’s get our detective hats on. We're looking for the reaction where things get tidier, where the molecules decide to settle down and get organized. It’s like finding the one person at the party who’s actually putting away the stray cups. A hero in their own right!
This isn't about memorizing complex formulas. It’s about intuition, about a gut feeling for how the universe tends to behave. It’s about recognizing that sometimes, order can emerge from what seems like a jumble.

The reactions we’re considering are like different flavors of a delicious, mind-bending dessert. Some are sweet and explosive, others are cool and collected. But only one will leave us with a sense of satisfying neatness at the molecular level.
Imagine you’re watching a documentary about nature. You see a flock of birds suddenly forming a perfect, swirling pattern in the sky. That’s a visual representation of decreased entropy – a collective movement towards order. We’re looking for that kind of organized beauty in our chemical reactions.
It’s a bit like observing a master craftsman at work. Every movement is precise, every piece fits perfectly. That’s what we’re hoping to see in our chemical transformations: a sense of purpose, a move towards a more structured state.
Now, the fun part is identifying which one does this. It’s not always obvious. Sometimes, the most surprising reactions are the ones that bring the most order. It’s a little scientific plot twist!
We’re not just looking for a change. We’re looking for a specific kind of change: a step towards less randomness, a move from a state of disarray to one of calm composure. Think of it as the universe taking a deep, organized breath.
This journey into decreased entropy is more than just chemistry. It’s a glimpse into the fundamental forces that shape our world. It’s about understanding why things happen the way they do, from the smallest atom to the grandest galaxy.

So, let’s consider our options. We have a few chemical scenarios laid out before us. Each one is a little experiment waiting to be understood. Our goal is simple: find the one that tidies up the most.
It’s like finding a perfectly organized bookshelf in a chaotic library. It stands out, it’s noticeable, and it’s a sign of something special happening. That’s what we’re after.
The beauty of this is that it’s relatable. We all understand the feeling of tidying up, of creating order. Chemistry just gives us a microscopic stage for this universal theme.
Consider the difference between a shuffled deck of cards and a perfectly stacked deck, sorted by suit and rank. The shuffled deck is high entropy, random. The stacked deck? That’s decreased entropy in action. We’re looking for that card-sorting reaction.
This isn’t just about abstract concepts. It’s about real transformations, real changes happening all around us, and even within us. And understanding them makes the world a more fascinating place.
The thrill comes from the discovery. From piecing together the clues and realizing, "Aha! That's the one!" It’s a moment of scientific enlightenment, a tiny spark of understanding.

So, when you look at these reactions, don't just see numbers and symbols. See the tiny dancers, the molecular ballet. See the potential for order to emerge from chaos.
It's a bit like watching a movie where the plot takes an unexpected turn towards resolution. Things that were scattered start to come together. It’s satisfying, it’s logical, and it’s what we’re hoping to find.
The reactions we’re examining are a spectrum of molecular behavior. Some are wild and free, others are more structured and deliberate. We are specifically searching for the deliberate ones, the ones that choose order.
Think about a snowflake forming. Each crystal is intricately structured, perfectly symmetrical. That’s a stunning example of nature creating order, decreasing entropy. We’re looking for a similar kind of elegant arrangement in our chemical reactions.
This journey into the heart of chemical reactions is an adventure. It’s about curiosity, about asking “why?” and then finding the beautiful, logical answers. And today, our big question is: which reaction is bringing the most order to the party?
The world of chemistry is full of surprises, and this quest for decreased entropy is one of the most delightful. It’s a reminder that even in the apparent randomness of the universe, there’s a constant drive towards structure and organization.
It's like a puzzle, where the pieces are molecules and the final picture is a state of perfect order. And we get to be the ones who figure out which reaction assembles that picture.
So, as we present our chemical scenarios, keep your eyes peeled for that touch of elegance, that moment of molecular tidiness. It’s a subtle but powerful force at play.
The satisfaction comes from understanding the underlying principles, from seeing how seemingly complex processes can be broken down into understandable patterns. And the pattern we’re looking for today is one of increasing order.
It’s not just about the reaction itself, but about the story it tells. The story of molecules finding their place, of chaos giving way to calm. And we’re here to read that story.
The challenge is to distinguish between reactions that simply change things and reactions that actively organize things. It’s the difference between a hurricane and a perfectly arranged garden.
So, which of these fascinating chemical events is the one that’s tidying up the most? Which one is showing us the universe’s knack for creating order? The answer lies within their molecular choreography. Get ready to be impressed!
