Which Of The Following Reactions Will Occur Spontaneously As Written

Alright folks, pull up a chair, grab a cuppa, and let's dive into something that sounds like it belongs on a very serious exam paper. We're talking about the age-old question: "Which Of The Following Reactions Will Occur Spontaneously As Written?" Now, before you start picturing me in a lab coat, looking all intense with bubbling beakers, let me assure you, we're keeping it light, breezy, and, dare I say, hilarious.
Think of spontaneous reactions like that friend who just shows up at your party uninvited, but, you know, in a good way. They just happen. No prompting needed. No awkward "please, would you mind terribly happening now?" It's the universe saying, "Yep, this is gonna go down."
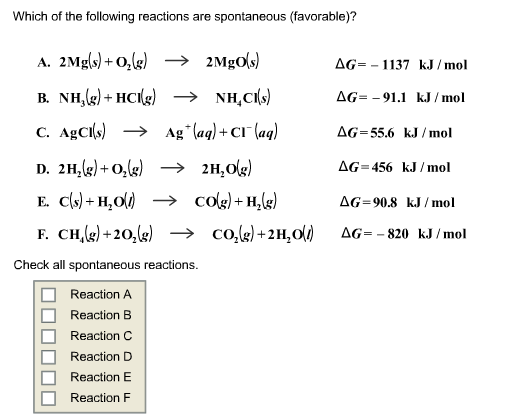
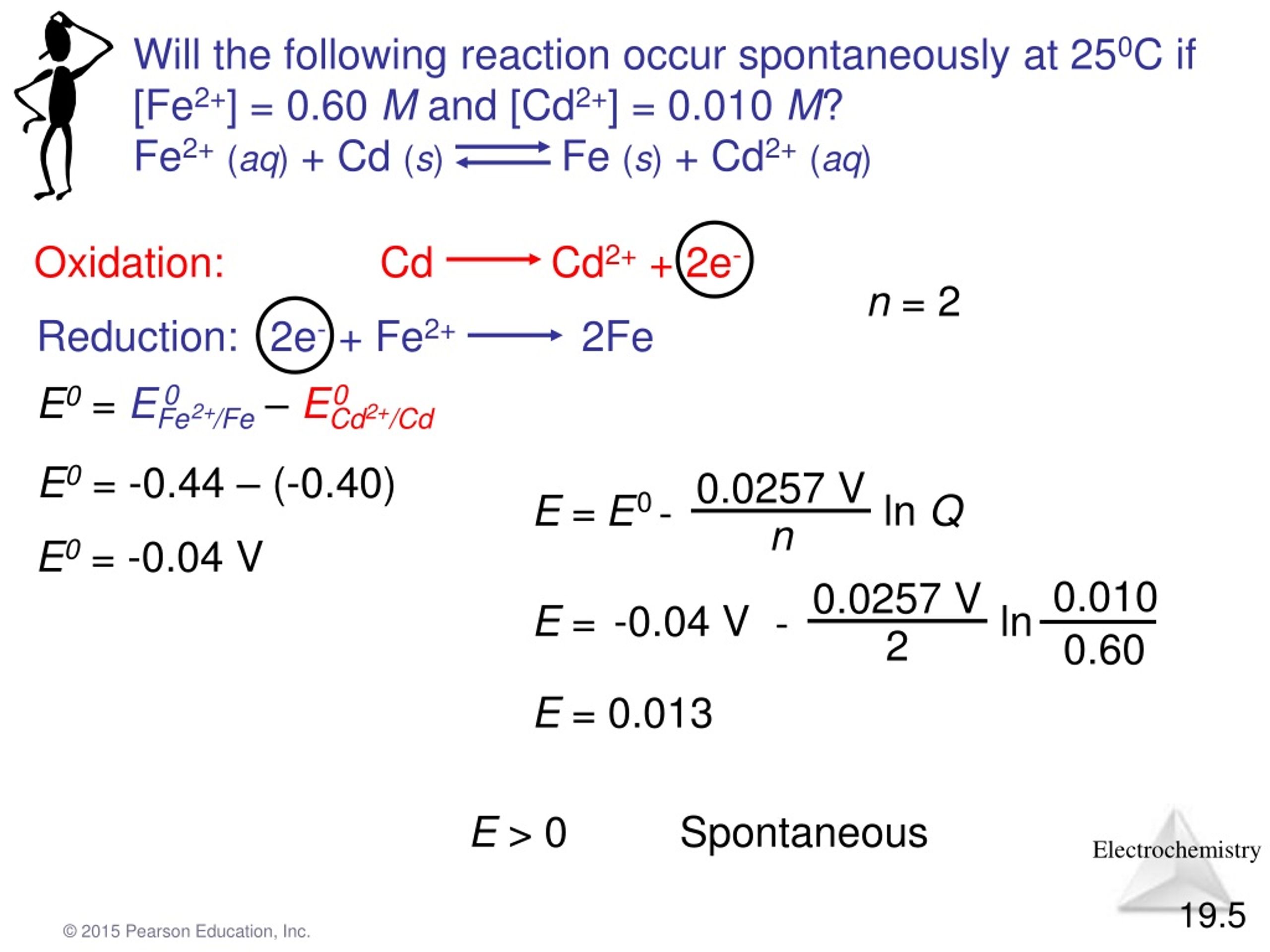
Now, the magic behind this whole spontaneous shindig is something called Gibbs Free Energy. Don't let the fancy name scare you. It's basically the universe's report card for a reaction. If the report card comes back with a nice, big, fat negative number, then BAM! Spontaneous. It's like getting an A+ on that pop quiz you totally forgot about. Sweet relief!
If that number is positive, though? Well, that's like your car refusing to start on a Monday morning. You can stare at it, you can plead with it, you can even offer it a tiny breakfast pastry, but it's just not gonna happen without a serious kick in the pants (or, you know, a jump start and a mechanic).
So, let's pretend we've got a few potential reactions lined up. Imagine them as contestants in a weird, microscopic talent show. Our job is to figure out which ones have that inherent 'oomph' to put on a show all by themselves.
Reaction Numero Uno: The Enthusiastic Exploder
Picture this: you've got two ingredients, let's call them 'Sparky' and 'Boomer'. Now, Sparky is all eager-beaver, and Boomer is just waiting for a nudge. When these two meet, they don't just gently mingle; they have a full-blown chemical fiesta! Think confetti cannons, a mariachi band, and maybe even a spontaneous outbreak of synchronized dancing. This reaction is practically begging to happen. Its Gibbs Free Energy is so negative, it's practically swimming in a pool of ink.
We're talking about things like, say, a piece of sodium metal hitting water. Sodium is like that super energetic puppy that just needs to be let off the leash. Water is like, "Oh, hi there!" and next thing you know, WHOOSH! A mini-fireworks display. Highly spontaneous, slightly terrifying if you're not expecting it, and definitely not something you want to try in your bathtub.
Reaction Numero Dos: The Hesitant Hug
Then we have our 'Cautious Carl' and 'Shy Shelly'. These two are like that awkward first date where everyone's trying their best, but there's a lot of fidgeting and avoiding eye contact. For them to get together, it's gonna take some serious effort. Maybe a bit of heat (that's the 'energy input' part), or perhaps a catalyst, which is like a smooth-talking wingman who encourages them to just, you know, talk to each other.
These reactions have a Gibbs Free Energy that's stubbornly positive. They're not saying "no," they're just saying "uh, maybe later?" or "only if you're buying." Think about turning graphite (that stuff in your pencil) into diamond. They're both just carbon, but graphite is comfy and doesn't want to change. Diamond is all fancy and expensive, and it needs a lot of pressure and heat to become that way. It’s not happening on its own while you're watching Netflix.
Reaction Numero Tres: The Indifferent Acquaintances
And finally, we have 'Just-So-So Sam' and 'Meh Mary'. These two can exist next to each other perfectly fine, neither really bothering the other. They're like colleagues who share an office but only exchange polite nods and occasional weather updates. Their Gibbs Free Energy is hovering around zero. It’s like they’re on the fence, neither leaning towards ‘yes’ nor ‘no’.
These are your equilibrium reactions. They’re doing a bit of both, forward and backward, at the same speed. It’s a delicate dance, a chemical tango. Think of a saturated solution of salt in water. You can keep adding salt, but eventually, it’ll just sit at the bottom, not dissolving, not precipitating much either. It's in a state of perfect, albeit boring, balance.
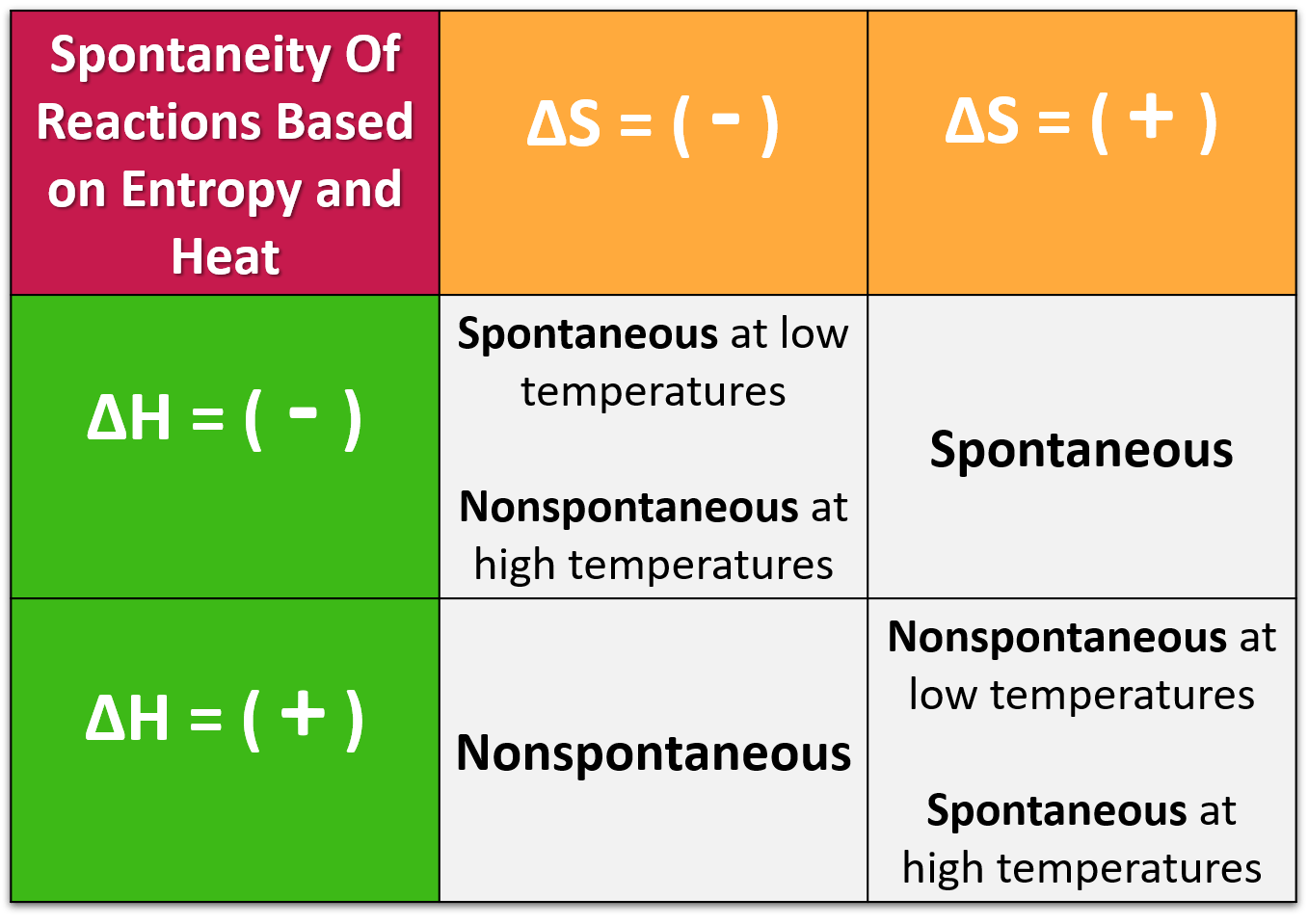
The Secret Sauce: Enthalpy and Entropy
So, what dictates this spontaneous dance? It's a dynamic duo: enthalpy and entropy. Enthalpy is like the energy 'give' or 'take'. If a reaction releases energy (exothermic, think warmth!), that's a big tick in the spontaneous box. It's like finally getting rid of that heavy backpack you've been carrying – pure relief!
Entropy, on the other hand, is all about disorder. The universe loves a good mess. If a reaction goes from an ordered state to a more disordered one (like ice melting into water, or a neatly stacked deck of cards getting shuffled), that's a big thumbs-up for spontaneity. The universe sees it and goes, "Ah, yes, that's more like it!" It’s like the joy of finding a forgotten ten-dollar bill in an old jacket – a delightful bit of unexpected chaos.
The Gibbs Free Energy equation, in its super simplified form, is basically saying: ΔG = ΔH - TΔS. Where ΔG is our friend Gibbs Free Energy, ΔH is enthalpy, T is temperature (because heat can really shake things up!), and ΔS is entropy. So, if enthalpy is nicely negative, or if entropy is super positive (especially at high temperatures), our ΔG is likely to be negative, leading to our beloved spontaneous reaction!
Putting It All Together: The Coffee Shop Verdict
When you're presented with a list of reactions and asked which will occur spontaneously, you're essentially looking for the one that the universe is least likely to complain about. Is it a reaction that releases a ton of energy and makes things messier? Bingo! Is it one that requires a huge energy input and tidies everything up? Probably not. Is it one that's just… chilling? Maybe, maybe not.

It’s like choosing your lunch at a café. A delicious, pre-made sandwich (spontaneous) is going to be consumed way faster than a raw chicken breast that you'd need to cook yourself (non-spontaneous). The sandwich just happens to be eaten. The chicken requires work.
So, the next time you see that question, don't panic. Just think about which reaction has the universe's blessing, which one is the eager beaver, the one that’s basically saying, "Let's do this!" And remember, even if a reaction isn't spontaneous on its own, a little encouragement (like heat or a catalyst) can sometimes convince even the most reluctant chemical couple to get together.
Now, if you'll excuse me, I think I've earned myself another cuppa. And who knows, maybe the act of me reaching for the kettle is a spontaneous reaction… in my book, anyway!
