Which Of The Following Is Correct Concerning Subatomic Particles

Ever feel like the universe is playing a giant game of "Where's Waldo?" with tiny, invisible stuff? You know, the really, really tiny stuff that makes up everything. We're talking about the building blocks of everything you can see, touch, and even smell. It's a wild world down there, way smaller than your average dust bunny.
So, when it comes to these super-duper small things, the subatomic particles, things can get a bit… perplexing. Scientists have names for them, like protons and electrons and neutrons. They sound important, right? Like characters in a superhero comic book, but with less spandex.
Now, imagine you're taking a quiz. A pop quiz, no less, about these teeny-tiny dudes. The question is, "Which of the following is correct concerning subatomic particles?" And then you have options. Oh, the pressure!
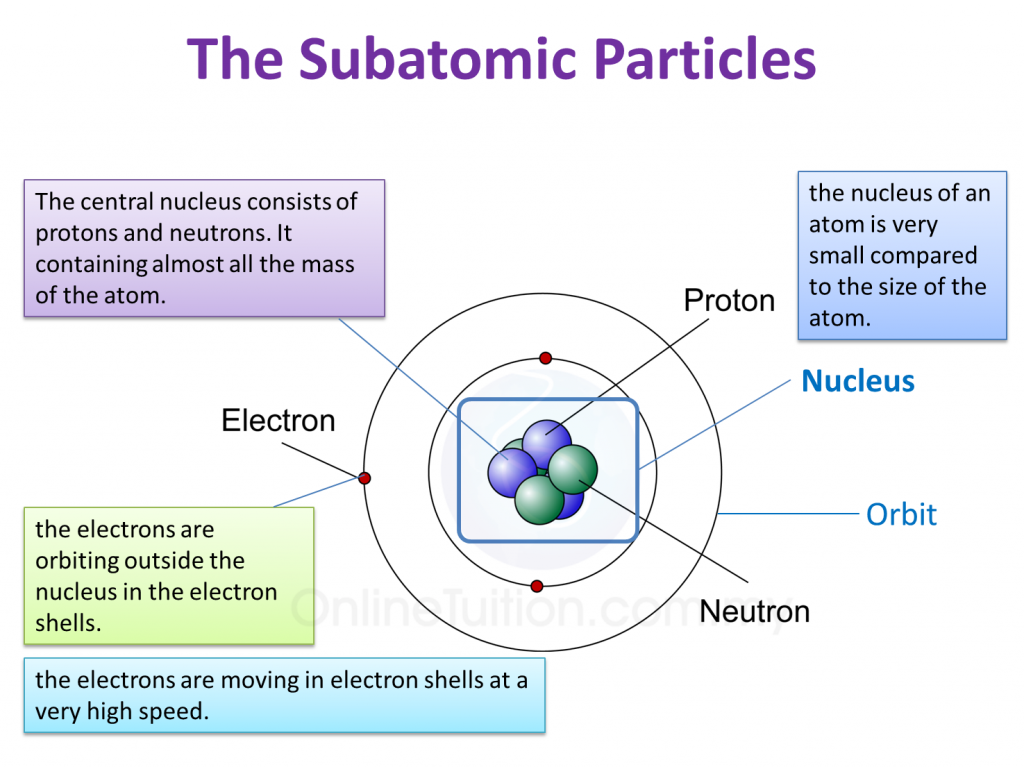
Let's think about the classic trio: protons, neutrons, and electrons. These are the VIPs of the atomic world. They hang out together, forming atoms, which are like tiny LEGO bricks for the universe. Without them, we'd just be… well, nothing.
Here’s where it gets fun. We have to figure out which statement is actually true. It’s like trying to decipher a secret code, but the code is written in quantum mechanics. And frankly, sometimes it feels like the instructions were written by a mischievous gnome who loves riddles.
Let’s consider the first option, perhaps one that says something about electrons being really, really heavy. Now, that sounds suspicious. I mean, I’ve dropped things before, and the heavier they are, the louder the thump. Electrons are so light, they practically float away on a gentle breeze. So, that option is probably a big fat nope.
Then, there might be a statement about protons having a negative charge. Hold on a minute! I always thought of protons as being the positive, upbeat ones. The ones that bring the good vibes. Like a ray of sunshine in the atomic nucleus. So, a negative charge for a proton? That feels wrong, like wearing socks with sandals on purpose.
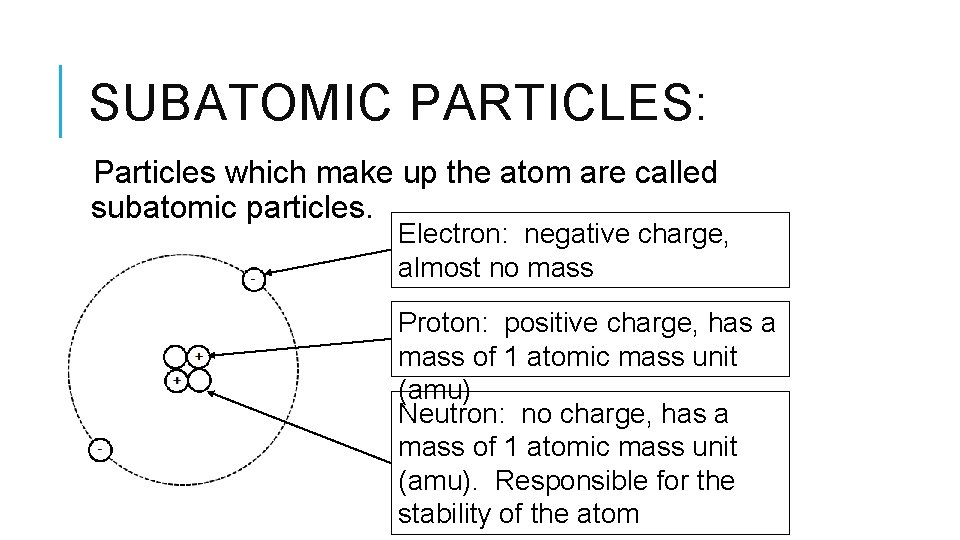
What about neutrons? They're supposed to be… well, neutral. Hence the name, right? Like the calm, collected ones at a party who just observe. So, if an option claims neutrons are buzzing with positive or negative energy, it’s probably fibbing. They’re the Switzerland of the subatomic world.
Now, let's say we get an option that mentions electrons having a negative charge. Ah, now we're talking! These little guys are the rebels. They’re the ones zipping around the nucleus, constantly on the go, and they’ve got that negative attitude. They're the tiny, energetic sparks that keep everything interesting.
And what if another option correctly states that protons have a positive charge? Bingo! These are the anchors. They’re the steady, reliable ones, providing the positive foundation for the atom. They’re like the grumpy but lovable grandpa of the nucleus.
And the neutrons? If an option says they carry no electrical charge, well, that’s spot on. They’re the quiet observers, the peacekeepers. They just hang out, adding mass without any of the electrical fuss. They’re the unsung heroes, really.
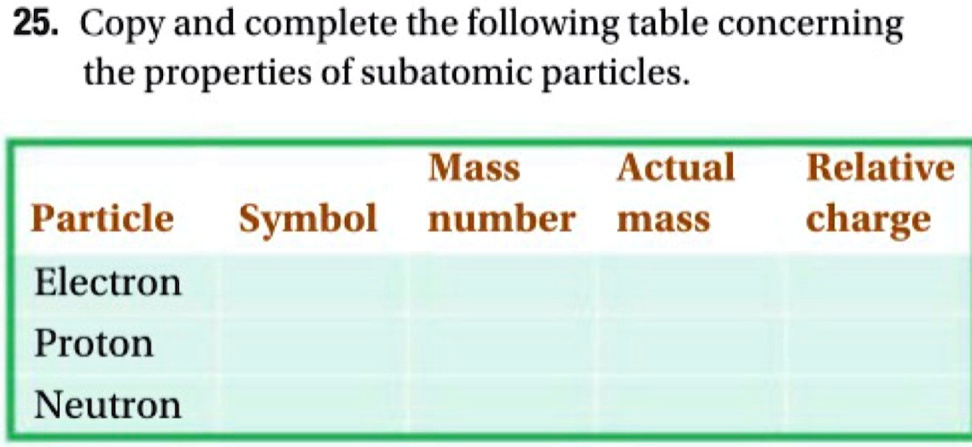
So, it seems like the correct statement is often the one that aligns with the basic, commonly accepted characteristics. Electrons are negative. Protons are positive. Neutrons are neutral. It's like knowing your ABCs, but for atoms. Simple, right? Except, the universe loves to throw curveballs.
But let's face it, sometimes the most obvious answers are the ones we second-guess. Especially when dealing with things we can't even see. It's like trying to guess what your cat is thinking – full of mystery and potential for hilarious misinterpretations.
Think about it. If you have to pick the correct one, you're looking for the statement that’s as true as gravity always pulling you down. The one that doesn't involve talking electrons or positively charged neutrons. Those are the red herrings, the scientific equivalent of a politician's promise.
So, the correct statement is usually the one that says: Electrons have a negative charge, protons have a positive charge, and neutrons have no charge. It’s the foundational knowledge. The stuff they teach you in those introductory science classes when you’re still awake.
It's almost an unpopular opinion to say that the most basic, textbook answer is often the right one in these quizzes. Because we want to believe there's some hidden trick, some complex nuance. But sometimes, it’s just about remembering the fundamentals.
The universe doesn't always need a dramatic plot twist. Sometimes, it's just a simple fact.
PPT - Unit 3 Review PowerPoint Presentation, free download - ID:5567417
So, when faced with that dreaded question about subatomic particles, take a deep breath. Remember the little guys: the zippy negative electrons, the steady positive protons, and the chill, neutral neutrons. They’re the foundation of our reality, and knowing their basic charges is like having a secret decoder ring for the cosmos.
And if you get it wrong? Well, blame it on the quantum fuzziness. Or the fact that subatomic particles probably have their own secret meetings where they decide to mess with us. Either way, it’s a good laugh.
Ultimately, the correct answer boils down to remembering the fundamental properties of these tiny titans. It’s about recognizing which statement accurately describes the electrical dance these particles perform. It’s not rocket science, even though they are involved in rocket science. It’s just particle science!
So, the next time you encounter a question about which statement is correct concerning subatomic particles, you'll know. It's the one that respects the charge of the electron, the proton, and the noble neutrality of the neutron. And that, my friends, is a universal truth.
It’s almost too simple, isn’t it? Like finding out the secret ingredient to your favorite recipe was just salt. But in the grand scheme of things, understanding these basic building blocks is pretty darn important. It's the difference between building a magnificent castle and a wobbly Jenga tower.
And let's be honest, who wants a wobbly Jenga tower of reality? Not me! I prefer my existence to be built on solid, scientifically sound principles. Even if those principles involve particles smaller than a speck of glitter on a fairy's wing.
So, to recap, the correct statement is the one that says: Electrons are negatively charged. Protons are positively charged. Neutrons have no charge. Simple. Elegant. And utterly crucial to the existence of everything you hold dear, including that cup of coffee you’re probably enjoying right now.
It's a beautiful thing, this order within chaos. The universe, in its infinite wisdom, has given us these fundamental rules. And sometimes, the most entertaining part is just remembering them.
So, go forth and impress your friends with your newfound subatomic particle knowledge. Or at least smile knowingly when someone struggles with the question. Because you, my friend, have cracked the code. The code of the tiny, the charged, and the neutral.
And that, my dear reader, is a victory worth celebrating. Perhaps with a cookie. Because even subatomic particles probably appreciate a good cookie, if they could only eat.

