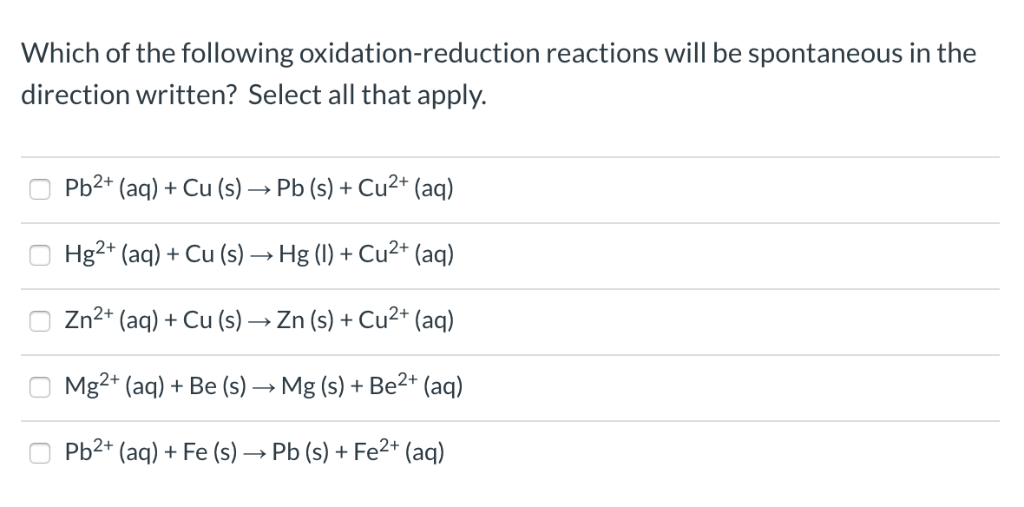
Which Of The Following Is An Oxidation-reduction Reaction Chegg
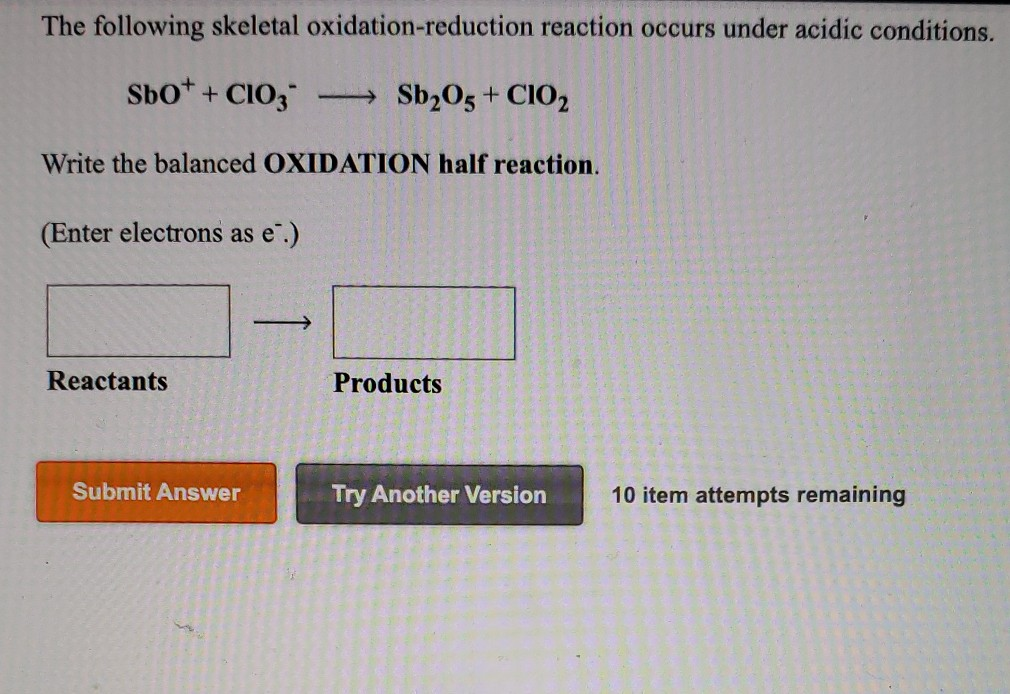
Hey there, curious minds! Ever stumbled upon a science question that made you go, "Huh? What's that all about?" Well, get ready, because we're diving into something that sounds a little fancy but is actually super cool. We're talking about the thrilling world of oxidation-reduction reactions, or as the internet wizards at Chegg sometimes put it, "Which of the following is an oxidation-reduction reaction?"
Now, before your eyes glaze over, let's break down why this is more exciting than it sounds. Think of it like a chemical soap opera. You've got these tiny little things called electrons. They're like the hot commodities in the chemical world. In an oxidation-reduction reaction, these electrons are playing a game of pass the parcel, but with a lot more drama.
So, what makes a reaction an oxidation-reduction reaction? It's all about the transfer of electrons. One chemical buddy is getting a little too clingy and gains electrons, while another is happily letting go and loses electrons. It’s a perfect give-and-take. The one losing electrons is called oxidized, and the one gaining them is called reduced. Don't worry about remembering those big words right now; just picture the electron swap!
Imagine you've got two friends. One has a super cool toy (an electron) and is tired of playing with it. The other friend really wants to play with that toy. So, they swap! The first friend, who gave away the toy, is now "oxidized" (they lost something). The second friend, who got the toy, is now "reduced" (they gained something). See? It's just chemistry with a bit of friendly (or maybe not-so-friendly) exchange.
Why is this so entertaining? Because these reactions are happening ALL THE TIME, all around you! Think about it: rust forming on your bike? That's oxidation-reduction. Your phone charging? Yep, oxidation-reduction. Even your own body digesting food? You guessed it – oxidation-reduction! It's the secret engine of so many processes we take for granted.
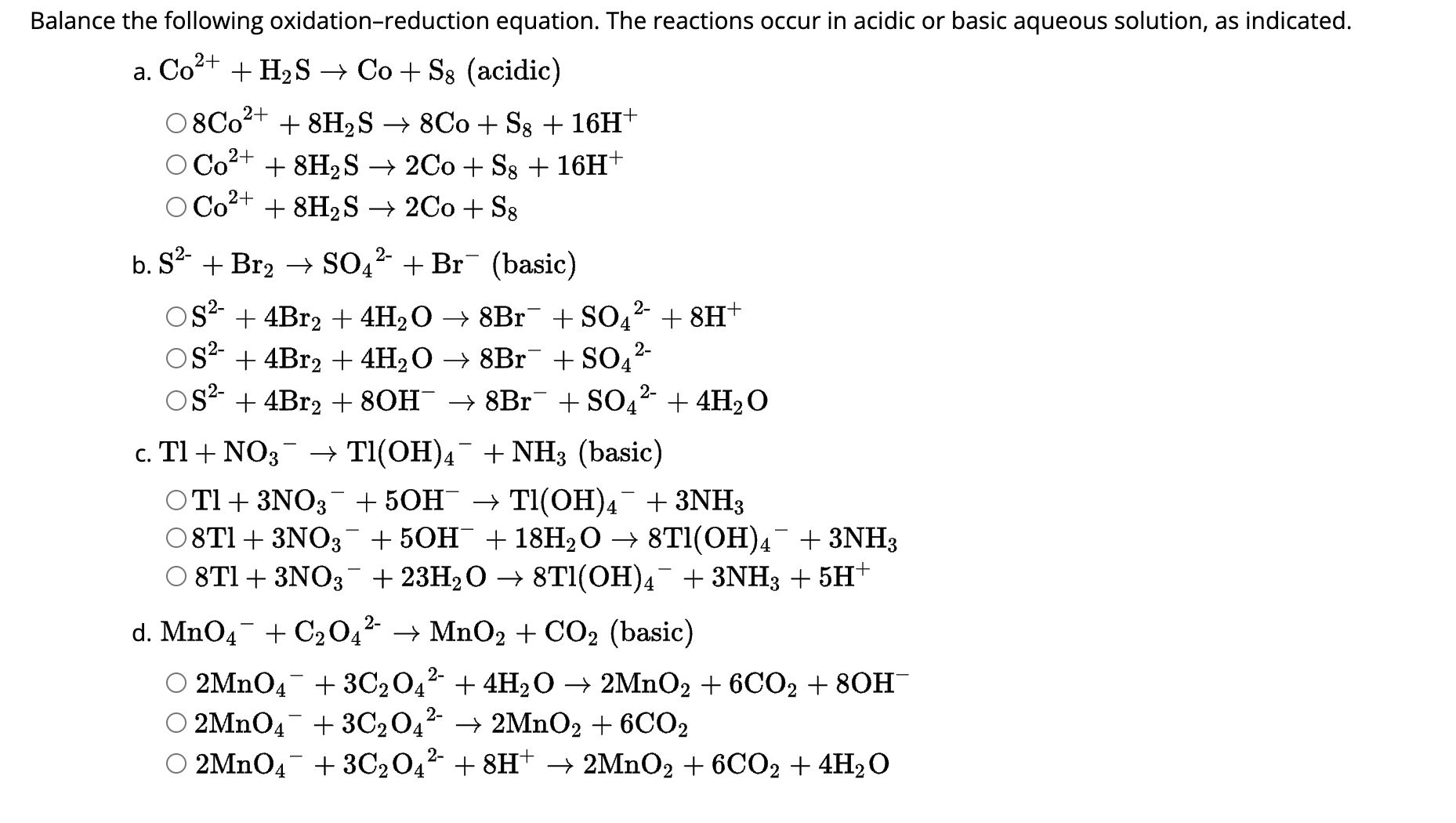
And when you see a question like "Which of the following is an oxidation-reduction reaction?" on a platform like Chegg, it’s like a treasure hunt. You're presented with a few chemical scenarios, and your job is to spot the one where electrons are doing their electrony dance. It’s a little puzzle, a brain teaser that makes you feel super smart when you crack it.
What makes it special is that it’s not just about memorizing formulas. It’s about understanding a fundamental concept that unlocks so many other chemical ideas. Once you get the hang of oxidation-reduction, you start to see the connections everywhere. It’s like learning a secret code that helps you understand the language of chemistry.
Let's say you're looking at a problem. You might see something like:
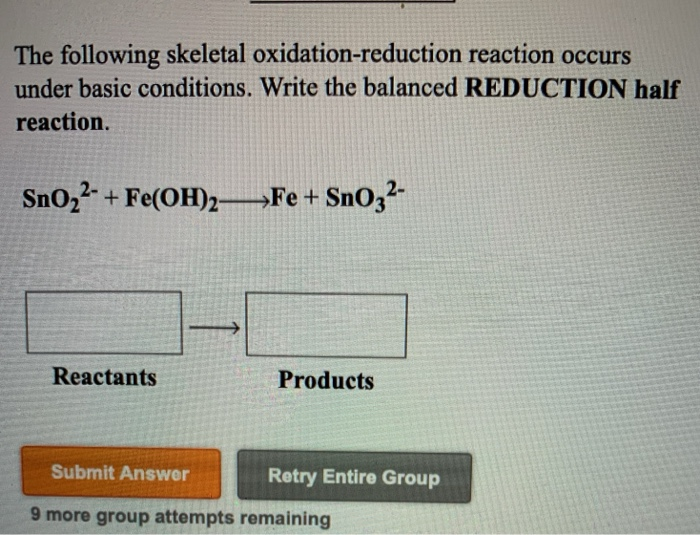
Which of the following is an oxidation-reduction reaction?
A) HCl + NaOH → NaCl + H₂O
Solved Which of the following oxidation-reduction reactions | Chegg.comB) 2Mg + O₂ → 2MgO
C) CaCO₃ → CaO + CO₂
Your mission, should you choose to accept it, is to pick the one where electrons are on the move. In option A, you've got a classic acid-base reaction. Things are rearranging, but not much electron shuffling. Option C is a decomposition reaction, kind of like breaking a toy apart. But option B? Ah, now we're talking! Magnesium (Mg) is giving up its electrons to oxygen (O₂), forming magnesium oxide (MgO). That electron transfer is the hallmark of an oxidation-reduction reaction. So, B would be your winner!

The beauty of these problems, especially when presented in a straightforward way like on Chegg, is that they encourage you to think critically. You're not just copying an answer; you're actively analyzing the chemical transformations. It's engaging because it requires you to apply a concept, not just recall it. It’s like being a detective, looking for clues (electron transfers!) in the chemical world.
And the satisfaction you get when you correctly identify an oxidation-reduction reaction is pretty darn great. It’s a small victory, but it’s a victory that builds your understanding and confidence. It’s these little "aha!" moments that make learning science, even the seemingly dry parts, truly enjoyable.
So, next time you hear the term "oxidation-reduction reaction" or see a question asking to identify one, don't shy away. Embrace it! Think of it as an invitation to explore the energetic world of electron exchange, a fundamental dance that powers our universe. And if you’re ever stuck, remember those friendly folks at Chegg are there to help you unravel these chemical mysteries, one electron at a time. It's a fun journey, and who knows, you might just find yourself looking forward to the next chemical drama!