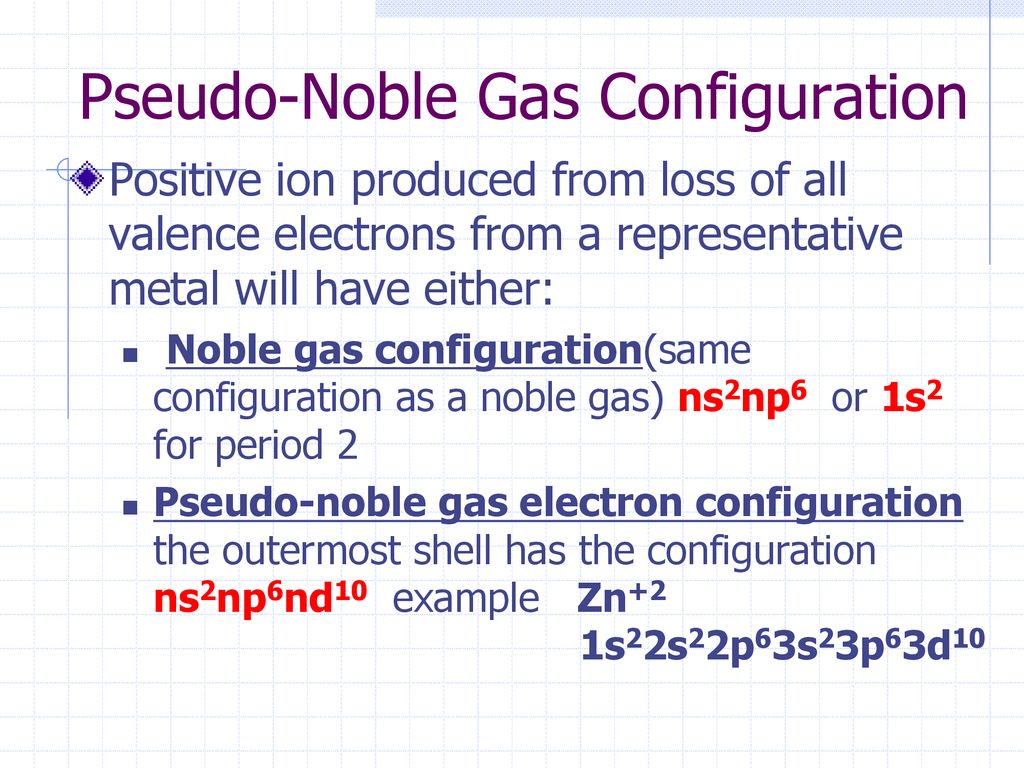
Which Of The Following Is A Pseudo-noble-gas Electron Configuration

Imagine you're at a cosmic party, and all the elements are mingling. Some are loud and boisterous, always looking to join in with others and make new connections. Others are a bit more reserved, perfectly happy chilling on their own. Then you've got the truly special guests, the ones who are just… content. They're the life of the party because they don't need anyone else to complete their happiness. These are our noble gases, the chillest dudes in the entire periodic table.
Think of them like your best friend who already has everything they could ever want. They've got the perfect playlist, the comfiest couch, and snacks galore. They're not looking to borrow anything or make any big plans. They're just… complete. Elements like Helium, Neon, and Argon are like this. Their outer electron shells are totally, blissfully full. It’s like they’ve got a full house of electrons, and there isn't an empty room in sight. This makes them super stable and incredibly reluctant to interact with anyone else. Why would they? They've already won the electron lottery!
Now, sometimes in this grand cosmic party, you meet someone who looks like they’re one of these super-content noble gases, but they’re not quite there. They’re like that friend who tells you they have everything, but you can subtly see they’re still checking their phone for notifications. They're not actually noble, but they've managed to arrange their electrons in a way that mimics that enviable, stable state. These are our pseudo-noble-gas configurations. They’re the imposters, the wannabes, the elements that are almost as cool as the real deal.
Let’s talk about how they pull off this clever trick. The real noble gases have a specific number of electrons in their outermost shell, like a perfectly balanced set of dominoes. They’re all lined up, full, and ready to do absolutely nothing. The pseudo-noble-gas configurators, however, have a slightly different, more complex arrangement. They might have a full outermost shell, but underneath that, they have a partially filled inner shell. It’s like having a perfectly tidy living room, but a slightly cluttered cupboard. It’s not a deal-breaker, but it’s not the absolute perfection of a noble gas.
Why do we even care about these pseudo-nobles? Well, they’re fascinating because they bridge the gap between the super-reactive elements and the super-stable ones. They’re the ones who might be persuaded to join in the fun, just a little bit. They’re not as eager to jump into chemical reactions as, say, an alkali metal trying to get rid of a single electron, but they’re more open than a true noble gas. They’re the element equivalent of someone who says, “I’m mostly fine staying in tonight, but if you really twist my arm…”
Consider the element Copper (Cu). It's a shining example, literally! Copper is used in everything from electrical wires to coins, and it’s got a bit of an identity crisis when it comes to its electron configuration. It tries to look like a noble gas. It has an electron configuration that's almost there. It’s like looking at a really good painting of a sunset, but you know it’s not the actual sky. Copper has a full outer shell, but it's got an inner shell that's not completely filled, making it almost as stable as a noble gas. This "almost" is what makes copper so useful! Because it's not perfectly stable, it's willing to participate in some chemical reactions, which is why it can form compounds and be used in all sorts of fascinating ways. It's not as reactive as some elements, but it's got a bit more pep than Helium.
Another intriguing character in this story is Silver (Ag). Similar to copper, silver also toys with the idea of being a noble gas. It flaunts a configuration that mimics that desired stability. It’s got that outer shell looking all neat and tidy, but there’s a little something-something going on in the shells beneath. This subtle difference allows silver to be a bit more reactive than a true noble gas. It’s this slight willingness to engage that makes silver so valuable for jewelry, mirrors, and even some medical applications. It’s not aloof like Neon, it’s got a little more personality!

The concept of a pseudo-noble-gas electron configuration is like discovering a secret handshake. It’s a way for an element to signal, “I’m pretty darn stable, but I’m not so exclusive that I won’t talk to you.” It’s this subtle, almost-noble characteristic that gives these elements their unique personalities and their crucial roles in the grand tapestry of chemistry. They’re not the aloof aristocrats of the periodic table, but they’re certainly not the boisterous commoners either. They’re the sophisticated diplomats, the ones who understand the value of a bit of stability without sacrificing all their charm.
So, next time you encounter an element, take a closer look. Is it a true, unadulterated noble gas, perfectly content in its own electron-filled universe? Or is it a clever pseudo-noble, just sophisticated enough to play the part while still having a little spark of reactivity? It’s a fun little game of chemical hide-and-seek, and understanding these “almost” noble gases gives us a whole new appreciation for the incredible diversity and subtle complexities of the elements that make up our world.
