Which Of The Following Is A Correct Monomer Polymer Pairing
Hey there, science enthusiasts and curious minds! Ever looked at your trusty plastic water bottle, your comfy fleece blanket, or even the chewy goodness of a gummy bear and wondered, "How did this all happen?" Well, get ready to have your world a little bit brighter, because we're diving into the wonderfully weird and surprisingly fun world of monomers and polymers!
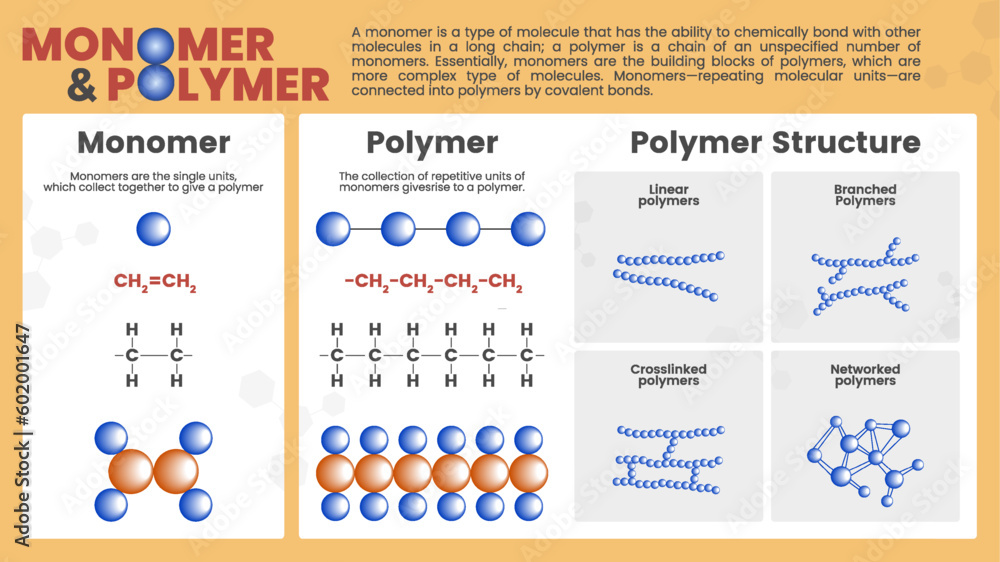
Now, don't let those fancy words scare you. Think of it like building with LEGOs, but on a super-duper, microscopic scale. We're talking about tiny building blocks that link up to make giant, amazing structures. Pretty cool, right? It’s the science behind so much of our everyday stuff, and understanding it can seriously unlock a new appreciation for the world around you. Plus, who doesn't love a good analogy?
Monomers: The Star Players!
So, what's a monomer? Imagine a single, eager LEGO brick. That's our monomer! It's the basic unit, the individual ingredient that, when given a little encouragement (usually a bit of heat, pressure, or a chemical nudge), decides to get together with its buddies and form something much bigger. They're the solo artists ready to join the band.
Think of them as the fundamental pieces of a puzzle. Each monomer has a specific shape and a desire to connect. And when they connect, oh boy, do they connect!
Polymers: The Superstars of the Show!
Now, when these monomers decide to hold hands, link arms, or basically form a giant, never-ending chain reaction, what do we get? We get a polymer! Polymers are the long chains made up of repeating monomer units. They're the completed LEGO castle, the finished symphony, the entire band rocking out!
These polymer chains can be incredibly long, sometimes made of thousands or even millions of monomer units all strung together. It's like a microscopic conga line that just keeps going and going!
The Big Question: Monomer Meets Polymer - The Right Pair!
The real magic happens when you have the correct monomer pairing. It’s not just about any old monomer linking up with any old thing. There’s a beautiful science to it, like a perfect match made in molecular heaven. We’re often asked, "Which of the following is a correct monomer-polymer pairing?" And let me tell you, it’s a question that can make you feel like a detective solving the case of the missing connection!

Let's break down some classic examples that are definitely, absolutely, spot-on pairings. Get ready to be amazed by the simplicity and the brilliance!
Glucose: The Sweetest Monomer.
First up, let's talk about glucose. If you've ever enjoyed a sugary treat or even just eaten something that gives you energy, you've encountered glucose. It's a simple sugar, a monosaccharide (that's just a fancy word for a single sugar unit). Glucose is like the fundamental unit of energy for many living things.
When multiple glucose molecules decide to link up, they form starch. Think of starch as the energy storage molecule in plants. It's the way plants stash away all that sweet, sweet glucose for later. So, the correct pairing here is glucose (the monomer) forming starch (the polymer). Pretty neat, huh? It’s the reason your bread and pasta keep you going!
But wait, there's more! Glucose can also link up to form cellulose. This is the structural backbone of plant cell walls. It’s what makes plants rigid and strong! So, glucose is a monomer that can form different polymers, like starch and cellulose. Isn't that wild? One little molecule, so many possibilities!
Amino Acids: The Protein Powerhouses.
Next on our monomer-polymer adventure are amino acids. These are the building blocks of life! Seriously, every protein in your body, from the ones that help you digest your food to the ones that make your hair shiny, is made from amino acids. There are 20 different common amino acids, each with its own unique personality.
When amino acids link together in a specific sequence, they form proteins. This is one of the most important monomer-polymer relationships in biology. Think of it like a chain of different colored beads, each bead being an amino acid. The order of the beads determines the final shape and function of the protein. So, amino acids (monomers) form proteins (polymers). Your body is basically a giant, constantly working polymer factory!
The beauty of proteins is their diversity. Just like you can build countless structures with different LEGO bricks, you can create an astonishing array of proteins with different combinations and sequences of amino acids. It’s a molecular masterpiece!
Nucleotides: The Blueprint of You!
Ready for the blueprint of life itself? Let's talk about nucleotides. These are the monomers that make up our genetic material: DNA and RNA. Each nucleotide has three parts: a sugar, a phosphate group, and a nitrogenous base.
When nucleotides link up in a long chain, they form nucleic acids. The most famous is DNA (deoxyribonucleic acid), which carries all the genetic instructions for building and operating an organism. Then there's RNA (ribonucleic acid), which plays various roles in gene expression. So, nucleotides (monomers) form DNA and RNA (polymers). How incredibly mind-blowing is that? The instructions for you are a polymer!

The sequence of these nucleotides in DNA is like a code, a secret language that dictates everything from your eye color to how your brain works. It's a testament to the power of these simple building blocks forming incredibly complex and vital structures.
Ethylene: The Plastic Puzzler.
Now, let's move to the world of man-made marvels. You've probably seen the recycling symbol on plastic items. Well, many of those plastics are polymers. A very common monomer that makes its way into our daily lives is ethylene. It's a simple hydrocarbon gas.
When ethylene monomers link up, they form polyethylene. This is one of the most widely produced plastics in the world! It's used in everything from plastic bags and films to bottles and toys. So, ethylene (monomer) forms polyethylene (polymer). That water bottle you're holding? Likely made from polyethylene!
It's amazing how a simple gas can be transformed into a material that's so versatile and essential to modern life. Of course, we all know the importance of recycling and using plastics responsibly, but understanding how they're made is a fascinating starting point.

Why Does This Even Matter? (Besides Being Super Cool!)
You might be thinking, "Okay, that's interesting, but how does knowing about glucose making starch make my day any better?" Well, my friends, it makes your day infinitely more interesting! It’s about seeing the interconnectedness of everything. It’s about realizing that the food you eat, the clothes you wear, even the air you breathe (in a very roundabout way!) are all products of these fundamental scientific principles.
This knowledge can spark a sense of wonder. It can make you look at everyday objects with newfound respect and curiosity. It's like discovering a secret language that the universe is speaking, and you're starting to understand it!
Plus, imagine the fun of impressing your friends with scientific tidbits! "Did you know your muscles are made of protein polymers formed from amino acids?" Boom! Instant science guru.
Embrace Your Inner Scientist!
So, the next time you encounter a plastic spoon, a piece of fruit, or even just a strong breeze (think about how plants use cellulose!), remember the monomers and polymers at play. The world is a giant, fascinating puzzle, and these tiny building blocks are the pieces that make it all fit together.
Don't stop here! This is just the tip of the iceberg. There are so many more incredible monomer-polymer pairings waiting to be discovered. Explore, ask questions, and let your curiosity run wild. The universe is literally made of these amazing connections, and understanding them is a truly inspiring journey. You've got this, future science superstar!
