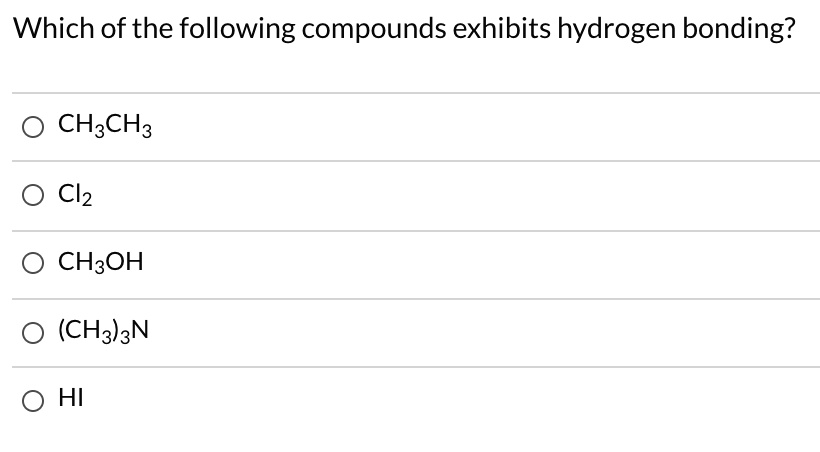
Which Of The Following Compounds Exhibits Hydrogen Bonding

Hey there, science explorer! So, you’ve stumbled upon the wonderful world of hydrogen bonding, huh? Don't worry, it’s not as complicated as it sounds. Think of it like a super-duper sticky hug that happens between certain molecules. Today, we're going to have some fun figuring out which of our special guests gets to participate in this molecular embrace. Grab a comfy seat, maybe a cup of your favorite beverage (tea, coffee, unicorn tears – whatever floats your boat!), and let's dive in!
First off, what exactly IS hydrogen bonding? Imagine you have a molecule, right? And in that molecule, you’ve got a hydrogen atom. Now, this hydrogen atom is feeling a little bit lonely because it's attached to an atom that's really, really good at hogging electrons. Think of it like that friend who always steals the best snacks at a party – super electronegative! We’re talking about atoms like oxygen (O), nitrogen (N), or fluorine (F). These guys are the electron-hoarding champions of the periodic table.
Because these electronegative atoms are so greedy, they pull the electrons closer to themselves, leaving the hydrogen atom feeling a bit… well, positively charged. It's not a full-blown, dramatic ion situation, but it's enough to give our little hydrogen a partial positive charge. We call this a polar bond, and our hydrogen is now the slightly-less-popular kid at the molecular dance, carrying a tiny bit of a positive vibe.
Now, enter another molecule. This molecule also has one of those electron-hogging, electronegative atoms (O, N, or F) hanging around, and guess what? That atom is probably rocking a partial negative charge because it's hogging electrons from its own partner. It’s like the opposite end of the dance floor, looking a bit negative and ready for some company.
And bam! When the partially positive hydrogen from one molecule gets close to the partially negative electronegative atom on another molecule, they form a hydrogen bond. It's like a magnetic attraction, a gentle but persistent pull that holds these molecules together. It’s not a full covalent bond (like the glue holding atoms together within a molecule), but it's stronger than your average attraction. Think of it as the molecular equivalent of holding hands really tightly.
So, Who Gets to Join the Hug Fest?
To be invited to the hydrogen bonding party, a molecule needs two crucial things:
- A hydrogen atom that is bonded to a highly electronegative atom (O, N, or F). This is our "donor."
- An electronegative atom (O, N, or F) on another molecule that has lone pairs of electrons (those are electrons hanging out on their own, not busy bonding). This is our "acceptor."
If a molecule has both of these features, it’s practically wearing a “Hydrogen Bond Enthusiast” t-shirt and is ready to mingle! If it’s missing either of these, well, it’s probably going to be sitting on the sidelines, observing the fun.
Let's Meet Our Contestants!
Alright, let's get down to business and look at some compounds. We're going to play a little game: "Does it Hydrogen Bond or Not?"
Contestant 1: Water (H₂O)
Ah, water! The universal solvent, the stuff of life, and a total hydrogen bonding superstar! Let’s break it down.
Water has a hydrogen atom. Check! Is that hydrogen atom bonded to a super electronegative atom? Yep, it's bonded to oxygen (O). Double-check! So, the hydrogens in water have a nice little partial positive charge. They're ready to be donors!

Now, does water have an electronegative atom with lone pairs? Absolutely! The oxygen in water is not only bonded to two hydrogens but also has two lone pairs of electrons. These are perfect acceptor spots! These oxygens are rocking those partial negative charges.
So, when one water molecule's hydrogen (partial positive) gets near another water molecule's oxygen (partial negative), poof! Hydrogen bond! This is why water has such a high boiling point, why ice floats (those crystal structures), and why it's so good at dissolving things. It’s all thanks to these awesome molecular hugs!
Contestant 2: Methane (CH₄)
Methane, the primary component of natural gas. Let’s see if it’s got the right stuff for hydrogen bonding.
Methane has hydrogen atoms. Check!
Are these hydrogen atoms bonded to a highly electronegative atom like O, N, or F? Nope. They are bonded to carbon (C). Carbon is electronegative, sure, but it’s not in the O, N, F elite club. It’s more like the friendly, slightly less popular kid. The difference in electronegativity between carbon and hydrogen isn’t big enough to create a significant partial positive charge on the hydrogen.
Because there’s no significant partial positive charge on the hydrogens, they can’t act as effective donors. And since carbon isn't an O, N, or F with lone pairs, there are no strong acceptor sites either.
So, methane is a no-go for hydrogen bonding. It’s more of a "socially distant" molecule in this regard.
Contestant 3: Ammonia (NH₃)
Ammonia! Smells… interesting, right? But is it good at hugging molecules?

Ammonia has hydrogen atoms. Check!
Are these hydrogens bonded to a highly electronegative atom? Yes! They are bonded to nitrogen (N). Nitrogen is one of our top-tier electron hoarders! This means the hydrogens in ammonia have a nice partial positive charge, making them excellent donors.
Does ammonia have an electronegative atom with lone pairs? You bet! The nitrogen in ammonia has one lone pair of electrons. This nitrogen is ready to be a fantastic acceptor for those positive hydrogens from other ammonia molecules (or even other molecules with suitable donors!).
So, ammonia molecules can definitely form hydrogen bonds with each other! This is why ammonia is a liquid at a much higher temperature than you’d expect for its size.
Contestant 4: Hydrogen Peroxide (H₂O₂)
Hydrogen peroxide, the stuff that cleans cuts (though maybe not the best idea anymore, but that’s a science lesson for another day!). Let’s look at its bonding abilities.
Hydrogen peroxide has hydrogen atoms. Check!
Are these hydrogens bonded to a highly electronegative atom? Yes! They are bonded to oxygen (O). Oxygen is a prime suspect for creating those partially positive hydrogens!
Does hydrogen peroxide have electronegative atoms with lone pairs? Absolutely! It has two oxygen atoms, and each oxygen atom has lone pairs of electrons. These oxygens are perfect acceptors!
Therefore, hydrogen peroxide can form hydrogen bonds. The hydrogen from one H₂O₂ molecule can bond with the oxygen of another H₂O₂ molecule, and vice-versa. It’s a real hydrogen bonding champ!
Contestant 5: Formaldehyde (CH₂O)
Formaldehyde, a common chemical used in labs and industry. Let’s see if it can get cozy.
Formaldehyde has hydrogen atoms. Check!
Are these hydrogens bonded to a highly electronegative atom? Yes, they are bonded to oxygen (O). This means the hydrogens will have a partial positive charge.
Does formaldehyde have an electronegative atom with lone pairs? Yes! It has an oxygen atom which has lone pairs of electrons. This oxygen is ready to accept those positively charged hydrogens.
So, a hydrogen atom from one formaldehyde molecule can form a hydrogen bond with the oxygen atom of another formaldehyde molecule. However, it's important to note that the hydrogen bond formed this way might be a bit weaker than in water or ammonia, as the overall polarity of the molecule and the distribution of electron density plays a role. But, technically, it exhibits hydrogen bonding!
Contestant 6: Hydrogen Sulfide (H₂S)
Hydrogen sulfide, known for its delightful "rotten egg" smell. Lovely. Let’s see if it’s good at holding hands.

Hydrogen sulfide has hydrogen atoms. Check!
Are these hydrogens bonded to a highly electronegative atom? They are bonded to sulfur (S). Now, sulfur is more electronegative than hydrogen, so there will be a slight partial positive charge on the hydrogen. However, sulfur is not in the O, N, F club. It’s less electronegative than those guys.
Does hydrogen sulfide have an electronegative atom with lone pairs? Yes, sulfur has lone pairs. However, because sulfur is less electronegative than O, N, or F, the hydrogen bond formed, if any, will be significantly weaker. In fact, for practical purposes and in introductory chemistry, H₂S is generally considered not to exhibit significant hydrogen bonding.
So, while there's a tiny bit of polarity, it’s not enough to qualify for the official hydrogen bonding club. Think of it as being on the waiting list, hoping for a chance to join.
The Verdict is In!
So, out of our contestants, who gets the gold star for hydrogen bonding?
- Water (H₂O): Absolutely! A hydrogen bonding champion.
- Methane (CH₄): Nope. Too evenly matched electron-wise.
- Ammonia (NH₃): Yes, indeed! Ready to form those bonds.
- Hydrogen Peroxide (H₂O₂): Definitely! It’s got all the right ingredients.
- Formaldehyde (CH₂O): Yes, it can! Though maybe not as strongly as some others.
- Hydrogen Sulfide (H₂S): Not really. The sulfur just isn't electronegative enough for a strong, defining hydrogen bond.
The key takeaway is that you need that special combination: a hydrogen attached to O, N, or F, interacting with an O, N, or F on another molecule. It’s like a molecular dating app where only specific profiles get a match!
Isn't science fun? It's all about understanding these subtle but powerful forces that shape the world around us. From the way water behaves to the intricate structures of life itself, hydrogen bonds play a starring role.
So, the next time you see water, or smell ammonia, or even just think about the molecules that make up your body, remember these invisible, yet incredibly important, connections. They are the silent architects of so much of what we experience. Keep exploring, keep questioning, and keep finding the wonder in every molecule. You’ve got this!
