Which Of The Following Combinations Cannot Produce A Buffer Solution

Hey there! So, you’re diving into the wonderful, and sometimes slightly bewildering, world of chemistry, huh? Specifically, we're gonna chat about buffers. You know, those unsung heroes that keep our pH levels from going totally haywire? Think of them like your best friend who always manages to smooth things over when things get a little too intense. Super useful stuff.
Now, the million-dollar question we’re tackling today is: Which of these combos cannot make a buffer solution? It’s like a little chemical puzzle, right? We’ve got a few options, and our job is to spot the odd one out, the one that just… won't cut the mustard. Ready to put on our detective hats?
Buffers: The Short & Sweet Version
Before we start eliminating, let’s just quickly recap what a buffer is. In a nutshell, a buffer solution is a magical mixture that resists changes in pH when you add either an acid or a base. How does it do this? Well, it's all about having a weak acid and its conjugate base, or a weak base and its conjugate acid. These two buddies hang out together, and when a rogue H+ or OH- tries to crash the party, they politely (or not so politely, depending on the situation!) neutralize it. Pretty neat, huh?
Think of it like this: Imagine you have a party, and your buffer is the bouncer. If someone shows up trying to start trouble (that's your added acid or base), the bouncer steps in. If it's too much acid, the conjugate base in the buffer steps up. If it’s too much base, the weak acid component is there to calm things down. It’s all about balance, people!
The key word here is weak. Strong acids and strong bases? They’re like the wild party-goers who don't listen to anyone. They’ll just dramatically change the pH, and your buffer won’t be able to do much about it. So, we’re always looking for those gentle giants, the weak ones.
Let’s Look at the Suspects!
Okay, so we've got some options to consider. The question is, which one doesn't fit the buffer recipe? This is where we need to get our chemistry goggles on and think about what makes a buffer tick.
Generally, a buffer solution is formed from:
- A weak acid AND its conjugate base (which often comes from a salt of that weak acid).
- A weak base AND its conjugate acid (which often comes from a salt of that weak base).
This is the golden rule. If you don't have this pairing, it's likely not going to be a buffer. Simple as that, right? Well, sometimes it's a little more subtle. Let’s break down why certain combinations work and others don't.
The Usual Suspects: What Does Work
Let's talk about some examples of actual buffer solutions. This will help us see what we’re aiming for.

Example 1: Acetic acid and sodium acetate.
Here, acetic acid (CH3COOH) is our weak acid. Its conjugate base is the acetate ion (CH3COO-). Sodium acetate (CH3COONa) is a salt that dissolves to give us those precious acetate ions. So, you’ve got your weak acid and its conjugate base? Bingo! That’s a buffer.
Example 2: Ammonia and ammonium chloride.
This time, we're playing with bases. Ammonia (NH3) is our weak base. Its conjugate acid is the ammonium ion (NH4+). Ammonium chloride (NH4Cl) is a salt that provides those ammonium ions. So, weak base and its conjugate acid? You guessed it – buffer!
See the pattern? It’s always that pair: weak acid/conjugate base or weak base/conjugate acid. They're like two peas in a pod, essential for buffering action.
The Red Herrings: What Doesn’t Work (Usually!)
Now, let’s get to the part where we identify the impostor. What kind of combinations would fail to produce a buffer solution? We need to think about what’s missing.
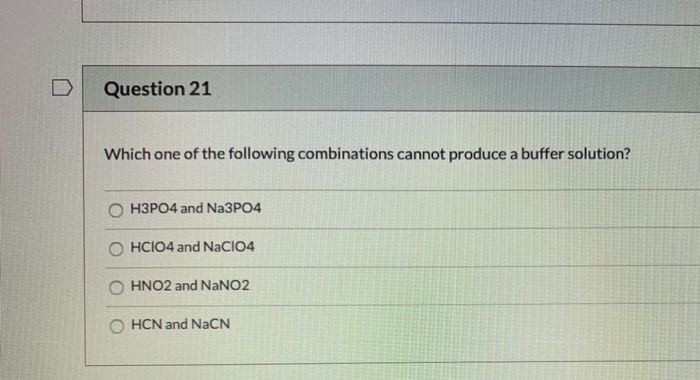
Scenario 1: A strong acid and its salt.
Let’s say you try to make a buffer with hydrochloric acid (HCl) and sodium chloride (NaCl). HCl is a strong acid. When you put it in water, it completely dissociates into H+ and Cl-. Now, NaCl dissociates into Na+ and Cl-. The problem? The chloride ion (Cl-) is the conjugate base of HCl, but because HCl is a strong acid, its conjugate base is super weak, practically non-existent in terms of its ability to accept protons. It’s like trying to make a team with a star player and a guy who just stands there. No buffering power!
So, a strong acid alone, or with its salt (where the anion is the conjugate base of the strong acid), just won't cut it. No weak acid component to soak up extra base, no conjugate base component to neutralize extra acid. It’s a one-trick pony, and the trick is just… being acidic.
Scenario 2: A strong base and its salt.
Similarly, if you try to make a buffer with, say, sodium hydroxide (NaOH) and sodium chloride (NaCl), you're going to be disappointed. NaOH is a strong base. It completely dissociates into Na+ and OH-. Again, NaCl gives Na+ and Cl-. The OH- is the part that wants to react, but there’s no weak acid there to accept it. And the Cl-? It’s not a weak base that can accept protons to buffer any added acid. It’s just… there. No buffering action here either. You've just got a basic solution.
Scenario 3: A weak acid and a strong base (or vice versa) in significant amounts.

This one is a bit trickier, but very important. If you mix a weak acid and a strong base, the strong base will react completely with the weak acid. For example, if you mix acetic acid (CH3COOH) with sodium hydroxide (NaOH), the NaOH will destroy most of the acetic acid, turning it into acetate ions (CH3COO-). You'll end up with a solution that is primarily sodium acetate and possibly some remaining acetic acid, or perhaps mostly water and sodium acetate if you added enough NaOH.
If you add the exact stoichiometric amount of strong base to a weak acid, you'll create the conjugate base, but you’ll have lost your weak acid component. You’ve effectively made the salt of the weak acid, which is great for a buffer if you also have the weak acid present. But without the weak acid, it's not a buffer. It's just a solution of the conjugate base.
Likewise, if you mix a weak base with a strong acid, the strong acid will react with the weak base, destroying it and creating its conjugate acid. Again, you'll be left with a solution that’s mostly the conjugate acid salt, but without the weak base to help things along, it's not a buffer.
The key is that both components of the buffer pair need to be present in appreciable amounts. If one component is completely consumed by the other, poof! No more buffer.
Scenario 4: Two strong acids or two strong bases.
This is a no-brainer, really. If you mix sulfuric acid (H2SO4) and nitric acid (HNO3), you just have a really, really acidic solution. There’s no weak acid or weak base to do any buffering. It’s like adding fuel to a fire. The pH will swing wildly with the slightest addition of anything else. Same goes for mixing two strong bases; you just get a really, really basic solution with no buffering capacity.

Scenario 5: A weak acid and a salt of a different weak acid.
Let’s say you have acetic acid (CH3COOH) and you mix it with sodium formate (HCOONa). Formic acid (HCOOH) is a weak acid, and its conjugate base is the formate ion (HCOO-). While you have a weak acid (acetic acid) and you have a conjugate base (formate ion), they are not the conjugate base of each other. The acetate ion is the conjugate base of acetic acid, and the formate ion is the conjugate base of formic acid. They can’t effectively buffer each other. They're like two friends who are good at their own thing, but they don't really have a symbiotic relationship to handle external pressures together. So, this combination typically won't form a buffer solution.
It’s like having a locksmith and a plumber show up at the same house to fix a leaky faucet. They're both skilled, but their skills aren't directly complementary for that specific problem. For buffering, you need the weak acid and its own conjugate base.
Putting It All Together: The Big Reveal
So, when you’re faced with a multiple-choice question asking which combination cannot produce a buffer solution, you’re looking for one of these scenarios:
- A strong acid + its conjugate base (because the conjugate base is too weak).
- A strong base + its conjugate acid (because the conjugate acid is too weak).
- A situation where a strong acid/base completely neutralizes a weak acid/base, leaving only one component behind (and not in the right ratio).
- Two strong acids or two strong bases.
- A weak acid/base mixed with the salt of a different weak acid/base.
The key is to always go back to the definition: weak acid + conjugate base OR weak base + conjugate acid. If your combination doesn't have this essential pairing in the right form, it’s probably not going to be a buffer. It’s like trying to bake a cake without flour – you’re missing a fundamental ingredient!
Remember, chemistry is all about these little rules and exceptions. But once you grasp the core concepts, it all starts to make sense. So, next time you see a bunch of chemical names, just ask yourself: “Do I have my weak acid and its trusty sidekick, the conjugate base, or my weak base and its equally trusty sidekick, the conjugate acid?” If the answer is a resounding yes, you’ve likely got a buffer. If it's a big fat no, well, then you’ve found your answer!
Keep practicing, keep asking questions, and don’t be afraid to get your hands (figuratively, of course!) dirty with some chemistry. You’ve got this!
