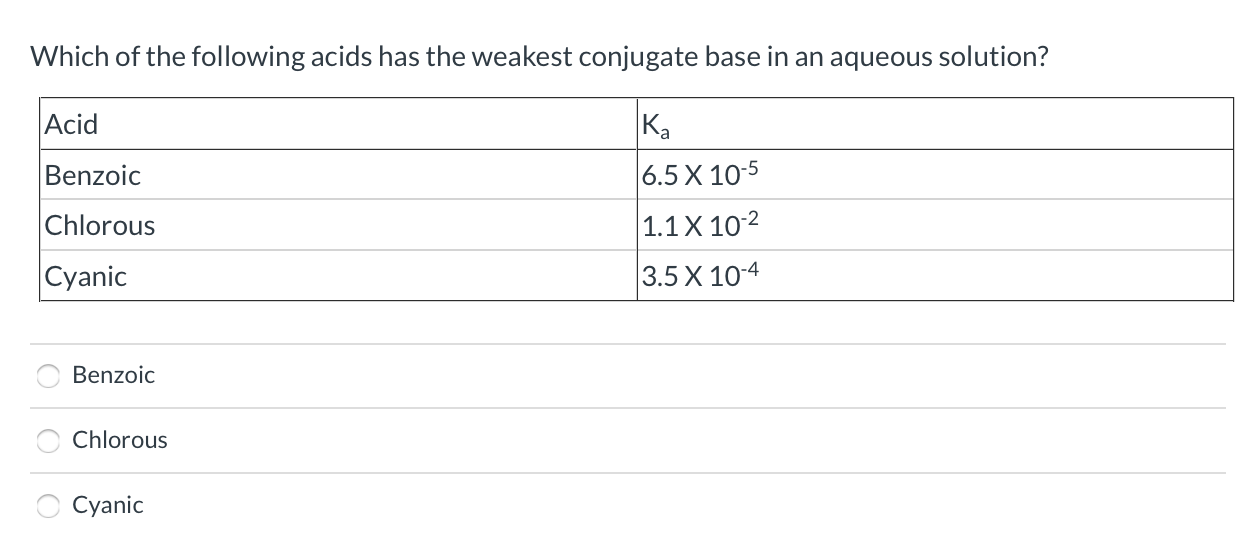
Which Of The Following Acids Has The Weakest Conjugate Base
Alright, settle in, grab your latte, and let's talk about something that might sound a little… well, acidic. But trust me, it's going to be way more entertaining than that time your uncle explained cryptocurrency at Thanksgiving. We're diving into the wacky world of acids and their conjugate bases. Sounds fancy, right? Think of it like this: acids are the dramatic ones, always ready to shed a proton (that's a tiny, positive little dude). And their conjugate bases? They're what's left behind after all the drama. And today, we're on a quest, a noble pursuit, to find the acid with the weakest conjugate base.
Imagine a party. The acid is the guest who loudly declares they're "leaving" every five minutes, but never actually goes. They're always donating something, usually a proton. Their conjugate base is the slightly bewildered person still standing there after the acid's done their whole spiel. Now, a strong acid? That's the person who dramatically throws their coat on the floor and storms out, leaving nothing behind but an echo and a faint scent of regret. Their conjugate base is basically a ghost, barely there. A weak acid, on the other hand, is like someone who mutters, "Fine, I'll consider leaving," and then just shuffles around the edges. Their conjugate base is still pretty much hanging out, feeling a bit clingy.
So, what does it mean for a conjugate base to be weak? It means it’s not very good at snatching back that proton if it gets offered again. It’s like a cat that’s had enough of your attention and is politely, but firmly, refusing to be petted. It's chill. It's independent. It's, dare I say, stable. The stronger the acid, the weaker its conjugate base. It's a cosmic balance, like a see-saw where one side goes up, the other goes down. No, seriously, it’s an inverse relationship. Mind. Blown. Or maybe just slightly tickled.
Now, you might be thinking, "This is all well and good, but where's the actual science? Where are the funny-sounding chemicals?" Fair enough! Let's get down to business. Imagine we have a lineup of potential contestants. We've got our usual suspects, the heavy hitters, and then some more… shall we say, reserved characters. We're looking for the acid that, after it's done its proton-donating thing, leaves behind a conjugate base that’s about as eager to grab another proton as a sloth is to run a marathon.
Let's meet our contenders. We've got, for example, hydrochloric acid (HCl). This guy is the absolute king of dramatic exits. He practically rips the proton out and throws it across the room. His conjugate base, the chloride ion (Cl⁻), is so chill, it’s practically horizontal. It has almost zero desire to get that proton back. It's a true ghost of a base. So, HCl? Definitely a strong acid, meaning its conjugate base is super weak. That's like… the gold standard of weak conjugate bases.
Then we have something like acetic acid (CH₃COOH), the stuff in vinegar. This is our weak acid friend. It's more like, "Oh, you want my proton? Sigh. Fine. Here." It's a bit hesitant. Its conjugate base, the acetate ion (CH₃COO⁻), is a bit more… present. It’s not exactly jumping at the chance to get a proton back, but it’s not entirely opposed to the idea either. It’s like a friend who says, "No, no, you go ahead," but is secretly hoping you'll change your mind.
We could also throw in water (H₂O). Water can be an acid, believe it or not! When it donates a proton, it becomes the hydroxide ion (OH⁻). Now, the hydroxide ion? It's a pretty decent base. It likes protons. So, water as an acid? Not super strong, and its conjugate base (OH⁻) is a respectable base, meaning it’s definitely not weak.

And then, there's the acid that makes even HCl look like a nervous amateur: perchloric acid (HClO₄). This acid is so strong, it’s practically an acid superhero. It gives up its proton with the enthusiasm of a lottery winner giving away free money. Its conjugate base, the perchlorate ion (ClO₄⁻), is so incredibly stable, it’s practically inert. It’s the ultimate chill-out artist of the chemical world. It has virtually no attraction to protons.
So, the question is, "Which of the following acids has the weakest conjugate base?" And if our list of options were, say, HCl, acetic acid, water, and perchloric acid, the answer would be screaming at you from across the room, probably while wearing a neon sign: Perchloric acid (HClO₄)!

Why? Because perchloric acid is one of the strongest acids out there. The stronger an acid is, the more readily it donates its proton. This means that once it has donated that proton, the resulting conjugate base is left in a state of extreme stability. It's like winning the chemical lottery and deciding you're never going back to work. The perchlorate ion (ClO₄⁻) is so stable, it's practically given up on life… in a good way, chemically speaking. It has almost no tendency to grab a proton back and become perchloric acid again. It's the ultimate surrender, the ultimate acceptance of its post-proton-donation existence.
Think of it this way: strong acids are like the person who breaks up with their partner for their partner, saying, "We're just not meant to be together, and you're better off without me." Their ex (the conjugate base) is left feeling a bit stunned, but ultimately free and unlikely to get back together. Weak acids are more like the couple who argues constantly but never actually breaks up. Their conjugate bases are still pretty invested in the relationship. So, when we're talking about the weakest conjugate base, we're looking for the acid that’s the most eager to get rid of its proton, leaving its counterpart in a state of blissful, proton-less independence.
So, to recap: Strong acid = extremely weak conjugate base. Weak acid = relatively strong conjugate base. It's a dance, a chemical tango, where the more one partner leads, the more the other gracefully retreats. And in the grand ballroom of chemistry, perchloric acid is leading the charge, leaving its perchlorate ion to waltz serenely into a life of proton-free tranquility. Now, wasn't that more fun than calculating molarity?
