Which Molecule Or Compound Below Contains A Polar Covalent Bond
/PolarConvalentBond-58a715be3df78c345b77b57d.jpg)
Okay, let's talk about molecules. You know, those tiny little building blocks of everything. We're going to play a little game today. Think of it like a molecular scavenger hunt, but way less dusty and much more exciting. We're on the lookout for a very special kind of connection.
We're searching for a molecule that has a polar covalent bond. Sounds fancy, right? Don't worry, it's not as intimidating as it might seem. It's like a slightly uneven tug-of-war happening between atoms.
Imagine two atoms holding hands. In a regular covalent bond, they're sharing their toys pretty equally. They're best buds, sharing nicely. But in a polar covalent bond, one atom is a little bit greedier, or maybe just a tiny bit stronger.
This atom pulls the shared electrons a little closer. So, one atom ends up with a slightly negative charge, and the other ends up with a slightly positive charge. It's like one kid getting a little more screen time, while the other is left waiting.
The Mystery Molecules
Now, we have a few contenders for our polar bond trophy. Let's meet them! We have some familiar faces and maybe a couple you haven't thought about in a while. Get ready to meet the suspects.
First up, we have water. Yep, good old H₂O. It's the stuff of life, and apparently, it's got some drama going on. You drink it, you swim in it, you spill it – and it's got a secret life.
Then there's methane. That's CH₄, the main ingredient in natural gas. It's what keeps your stove lit and your house warm. It's pretty chill, or is it? We need to investigate.

Next, we have carbon dioxide. CO₂. We breathe it out, plants breathe it in. It's a big player in our atmosphere. Does it have any internal squabbles?
And finally, let's throw in molecular oxygen. O₂. Just two oxygen atoms chilling together. They're the same, so what could possibly go wrong? We’ll see.
Unpacking the Bonds
Let's start with our friend water. It's made of one oxygen atom and two hydrogen atoms. Oxygen is a bit of a diva. It really likes to hog those electrons.
So, the oxygen atom in water pulls the electrons from the hydrogen atoms towards itself. This makes the oxygen end of the water molecule a little bit negative. Think of it as the oxygen being the popular kid, and the hydrogens are just happy to be in its orbit.
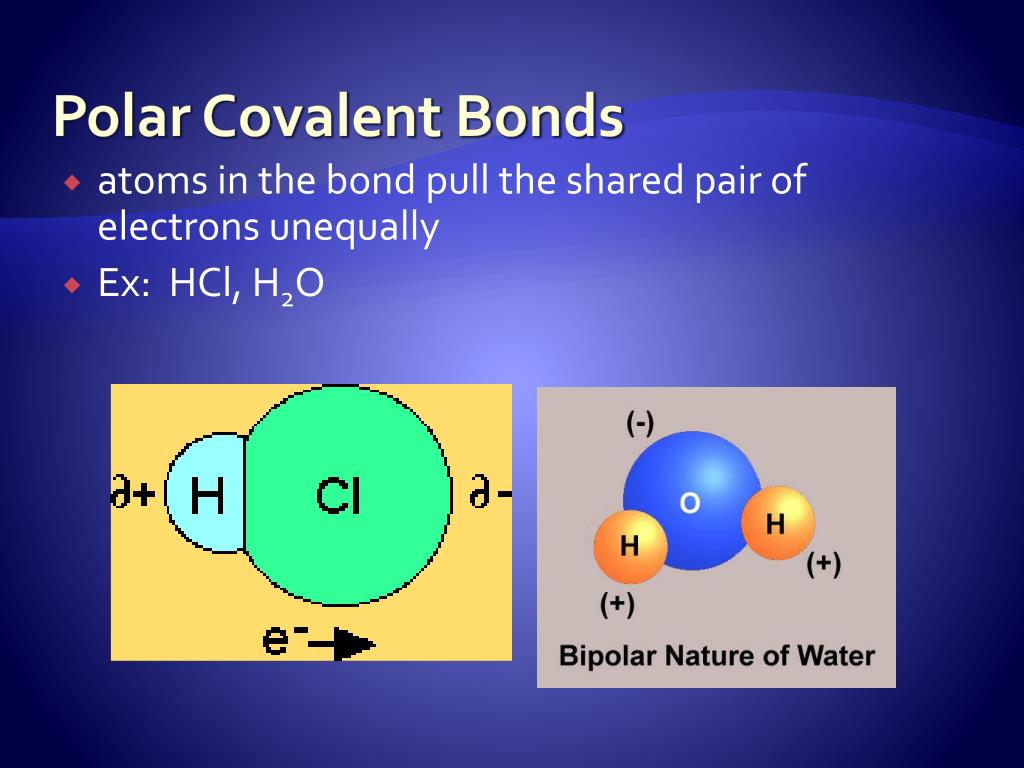
The hydrogen atoms, on the other hand, are left with a slightly positive charge. They're like the shy friends who don't get as much attention. This difference in charge is exactly what makes the bonds in water polar covalent. It's a bit uneven, but it works!
Now, let's look at methane. It's a central carbon atom with four hydrogen atoms. Carbon is pretty fair-minded. It shares its electrons pretty equally with the hydrogens.
There's no real tug-of-war happening here. The electrons are shared nicely, like a group of friends passing around a board game. So, the bonds in methane are nonpolar covalent. They're as balanced as a perfectly constructed Jenga tower.
Moving on to carbon dioxide. This one has a central carbon atom bonded to two oxygen atoms. You might think, "Oh, oxygen again, it must be polar!" But here's where things get interesting.
While the individual C-O bonds are indeed polar (oxygen pulls those electrons!), the molecule is perfectly symmetrical. It's like two equally strong people pushing on opposite sides of a door. The pushes cancel each other out.
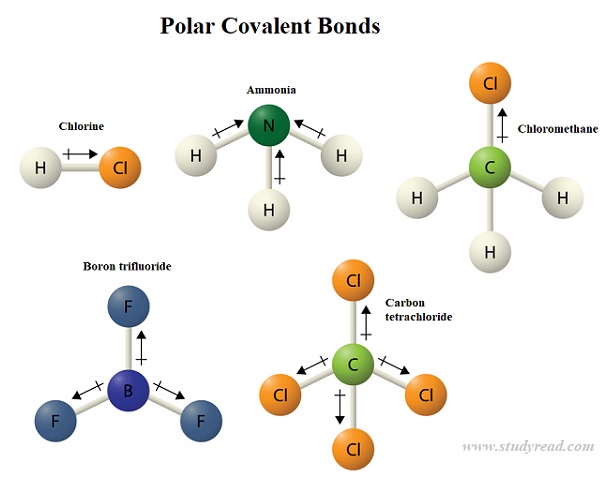
So, even though there are polar bonds within carbon dioxide, the molecule as a whole is nonpolar. The unevenness cancels out, leaving everything surprisingly balanced. It’s a bit of a trickster molecule.
Finally, we have molecular oxygen, O₂. It's just two oxygen atoms bonded together. Since they are the same element, they have the exact same pull on electrons.
There's no advantage for one oxygen over the other. They're sharing the electrons perfectly equally. It's like two identical twins who can never decide who gets the bigger slice of cake because they're both just as happy.
Therefore, the bond in molecular oxygen is definitely nonpolar covalent. It's a bond of pure equality. No drama, no favoritism, just solid sharing.

The Verdict is In!
So, after our little molecular investigation, who is the winner of the polar covalent bond contest? Drumroll please...
It's WATER! H₂O takes the prize.
The uneven sharing of electrons between oxygen and hydrogen gives water its unique properties. It's why water molecules like to stick together, forming the basis for all life as we know it. It's a small, uneven bond with a big impact.
Methane, carbon dioxide, and molecular oxygen all have their own stories, but they don't have that signature polar covalent bond that makes water so special. They're more about sharing, less about the dramatic tug-of-war.
Isn't science fun? Sometimes the most important things are a little bit unbalanced. It’s our unpopular opinion, but we stand by it. Embrace the polar!
So next time you take a sip of water, give a little nod to that amazing polar covalent bond. It's doing a lot more for you than you probably realize. Cheers to the unevenly shared electrons!
