Which Missing Item Would Complete This Alpha Decay Reaction

You know, I was trying to teach my niece about atoms the other day. Picture this: a tiny, slightly bewildered 8-year-old staring at a whiteboard covered in squiggly lines and mysterious letters. I'd just explained that atoms are the building blocks of everything, and she, bless her cotton socks, looked like I'd just told her the moon was made of cheese. "So," she'd said, pointing a sticky finger at a diagram of a carbon atom, "this is like… a really, really small Lego brick?"
It was close enough, I thought. And then we got to the fun stuff: radioactive decay. I tried to explain how some atoms are, shall we say, a bit unstable. They’re like that one relative who’s always pacing the room before a big holiday – they’ve got too much energy and something’s gotta give. And sometimes, what gives is a whole chunk of the atom itself! It's called alpha decay, and it's pretty darn neat.
But here’s where it gets tricky, even for grown-ups. Imagine you're doing a puzzle. You've got most of the pieces, the picture is starting to form, but there's one missing. And without that one piece, the whole darn thing just doesn't make sense. That's kind of what it's like when we look at alpha decay reactions. We see what goes in, and we see most of what comes out, but there's that nagging feeling… something’s missing.
So, what exactly is alpha decay, you might be asking? Well, it's when a big, heavy, kind of grumpy atomic nucleus decides it’s had enough. It’s just too much going on inside, too many protons and neutrons jostling for space. So, to calm itself down, it spits out a tiny little package. This package isn't just a random bit of fluff; it's a specific combination of two protons and two neutrons, bound together. Anyone who’s played with atoms for a while will recognize this little duo immediately. It's what we call an alpha particle.
Think of it like a parent bird nudging a baby chick out of the nest. The parent atom is the big, lumbering bird, and the alpha particle is the chick taking its first flight. The parent atom is left with a slightly smaller, more stable nucleus. It’s basically shedding some weight to feel better. Simple, right? Well, almost.
The coolest part about alpha decay is that it's actually a pretty predictable process. We can write it out as a chemical equation, just like you might see in a chemistry textbook. On one side, you have the original, unstable atom (we call this the parent nuclide). On the other side, you have the new, slightly less unstable atom (the daughter nuclide) and the alpha particle that got yeeted out. Easy peasy.
But here’s where the puzzle analogy comes back. Sometimes, when scientists are looking at these reactions, they’ve got the parent nuclide and they've detected the daughter nuclide and the alpha particle. Everything looks like it adds up. But then, they scratch their heads and go, "Wait a minute… is that all?" It's like you've accounted for the bird and the chick, but you feel like there should be a worm in the picture somewhere, and it’s just not showing up.
The Case of the Missing Piece
So, let's dive into a specific example. We're going to talk about a hypothetical (but totally plausible!) alpha decay reaction. Imagine we're observing the decay of a super heavy, really unstable element. Let's call it Element X. Now, Element X is a bit of a drama queen. It's got a massive nucleus, bursting at the seams with protons and neutrons. It's so unstable, it needs to shed mass to achieve some semblance of nuclear peace.
We observe Element X undergoing alpha decay. We know that when an atom undergoes alpha decay, it loses 2 protons and 2 neutrons. This means its atomic number (the number of protons) decreases by 2, and its mass number (the total number of protons and neutrons) decreases by 4.
Let's assign some numbers. Suppose Element X has an atomic number of 110 and a mass number of 278. So, our parent nuclide is represented as $^{278}_{110}\text{X}$.
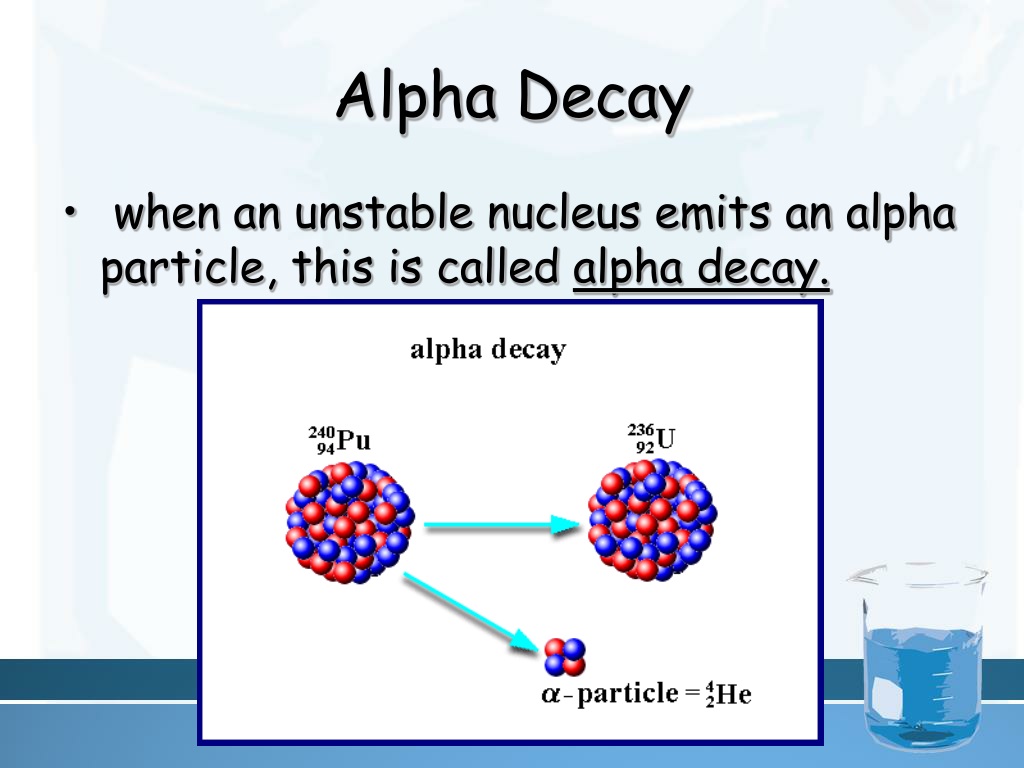
When it undergoes alpha decay, it emits an alpha particle. We know an alpha particle is essentially a helium nucleus, with 2 protons and 2 neutrons. So, it has an atomic number of 2 and a mass number of 4. We write it as $^4_2\text{He}$ or, more commonly in nuclear equations, as $\alpha$.
Now, the equation should look something like this:
$$^{278}_{110}\text{X} \rightarrow \text{Daughter Nuclide} + \alpha$$To find the daughter nuclide, we need to balance the atomic numbers and the mass numbers. The atomic number of the daughter nuclide will be $110 - 2 = 108$. The mass number of the daughter nuclide will be $278 - 4 = 274$.
So, the daughter nuclide would have 108 protons and 174 neutrons (since $274 - 108 = 166$, whoops, wait, that's $274 - 108 = 166$. Let me recheck my math... Ah, $278-4 = 274$. And $110-2=108$. So, the daughter nuclide has an atomic number of 108 and a mass number of 274. Phew, got there eventually! My brain sometimes feels like it’s undergoing its own kind of decay when I do quick arithmetic). This element with atomic number 108 is known as Hassium (Hs).
So, the expected alpha decay reaction is:
$$^{278}_{110}\text{X} \rightarrow ^{274}_{108}\text{Hs} + \alpha$$
On the surface, this looks perfectly balanced. The numbers add up. Protons on the left equal protons on the right. Mass numbers on the left equal mass numbers on the right. Hooray! Science!
But what if, in the lab, the scientists are seeing something else? What if they’re detecting $^{274}_{108}\text{Hs}$ and they’re detecting the alpha particle, but they're also noticing a bit of extra energy that doesn't quite fit? Or maybe, just maybe, there's a tiny bit of another particle they can't quite identify, a ghost in the machine of nuclear physics.
The Plot Thickens… (And Gets More Complicated)
This is where it gets interesting. Sometimes, alpha decay isn't quite as "clean" as the basic equation suggests. There are subtle nuances. And when we talk about a "missing item," it could mean a few different things in the context of nuclear reactions.
It could be that our initial assumptions about the parent nuclide or the daughter nuclide were slightly off. Maybe the experiment wasn't perfectly calibrated, or the detectors had a tiny bit of background noise that mimicked a signal. It happens! Science is messy, folks.
Or, it could be that there are secondary processes happening. Alpha decay is often accompanied by the emission of gamma rays. Gamma rays are high-energy photons, essentially packets of electromagnetic radiation. They're like the nervous energy left over after the alpha particle has left the building. The nucleus, having just undergone a big change, might still be in an excited state, and it sheds this excess energy in the form of gamma rays. So, if you’re only looking for particles, you might miss the energetic fireworks.
The equation then might look more like:
$$^{278}_{110}\text{X} \rightarrow ^{274}_{108}\text{Hs} + \alpha + \gamma$$
Where $\gamma$ represents the gamma ray. So, in this case, the "missing item" would be a gamma ray. It's not a chunk of matter, but it’s a crucial part of the energy balance.
But what if it's not just energy? What if the scientists are detecting a particle that shouldn't be there according to the simple alpha decay model? This is where things get really speculative and exciting. In some extremely rare or specific decay modes, other particles could be involved. Think of it like a magic trick where you expect a rabbit and a dove, but suddenly a hamster pops out of the hat too. Very unexpected!
So, What's the Real Missing Item?
Okay, let's get back to our original question: "Which Missing Item Would Complete This Alpha Decay Reaction?" If we're talking about the fundamental components of a standard alpha decay, and assuming our initial identification of the parent and daughter nuclides is correct, the most common "missing" piece that is often overlooked in simplified explanations is the gamma ray. It's the energetic exhale of the nucleus after it’s done the heavy lifting of spitting out an alpha particle.
However, the question is phrased in a way that suggests a material missing item, something tangible like a proton or a neutron. In the context of basic alpha decay, where the reaction is purely: Parent $\rightarrow$ Daughter + Alpha, there isn't a missing proton or neutron. If there were, the atomic and mass numbers wouldn't balance.
But, here’s a little thought experiment for you. Imagine the scientists are incredibly precise. They’ve accounted for all the mass, all the charge, all the energy. And yet, there’s a tiny discrepancy. It's the kind of discrepancy that makes physicists’ eyes light up. What could it be?
Perhaps the "missing item" isn't a fundamental particle in the way we usually think of it. Could it be something like an electron neutrino? Neutrinos are notoriously difficult to detect because they interact very, very weakly with matter. They're often produced in beta decay, but in some complex nuclear processes, they can be involved in other decays as well. They carry away energy and momentum, and if they weren't accounted for, they could manifest as a slight imbalance in the energy-momentum budget of the reaction. This is a bit more advanced, and not typically part of a basic alpha decay explanation, but it's the kind of thing that would make a physicist’s heart beat faster.
Or, and this is where we get into some really cutting-edge stuff, what if the "missing item" is a hint towards new physics? Imagine they observe a decay that doesn't quite fit any known particle or energy signature. It's an anomaly. This anomaly could be the first sign of a new fundamental particle, an entirely new force, or a deviation from our current understanding of the Standard Model of particle physics. That would be the ultimate missing item – the key to unlocking a new level of understanding about the universe!
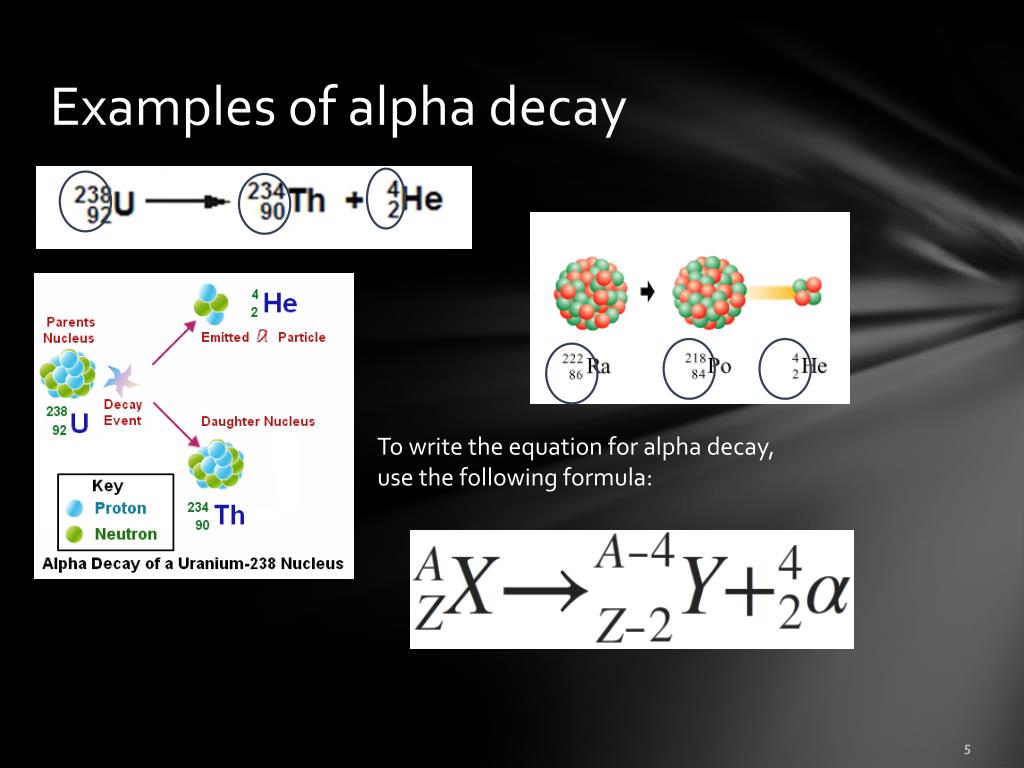
For the sake of keeping it somewhat grounded in typical nuclear physics curriculum, let's go back to the most probable "missing" component in a real-world observation that might not be immediately obvious from a simple equation. If the reaction equation for alpha decay is written as:
$$^{A}_{Z}\text{X} \rightarrow ^{A-4}_{Z-2}\text{Y} + ^4_2\text{He}$$And all these components are accounted for, but there's still an energy deficit or a momentum imbalance, then the most likely missing item that carries energy and momentum but is hard to detect is a neutrino. Specifically, in alpha decay, if there’s any deviation from the simplest decay path, a neutrino might be involved. However, standard alpha decay itself doesn't necessarily produce neutrinos. They are much more common in beta decay.
But if the question implies a deviation from the expected simple alpha decay, and we're talking about particles, then the most plausible, though still rare in simple alpha decay, candidate for a missing particle that carries away energy and momentum would be a neutrino. But it’s crucial to remember that standard alpha decay, as defined, does not intrinsically emit neutrinos. The alpha particle is the primary emission from the nucleus.
The Takeaway for Your Own Puzzles
So, to wrap this up, if you’re presented with a standard alpha decay equation and asked what’s missing, and you can clearly see the parent nuclide, daughter nuclide, and alpha particle, then you’re probably being tested on your understanding that gamma rays are often emitted alongside alpha particles to account for excess energy. They are a form of radiation, not a particle in the same sense as a proton or neutron, but they are a crucial component of the overall energy balance.
If, however, the scenario is more experimental, where something is detected that shouldn't be there, or a measurable quantity is unaccounted for, then the "missing item" could be a sign of more complex decay processes, or even, in the most exciting scientific discoveries, a hint of new physics. But for most of us, when we see a simplified alpha decay equation, the invisible guest that often completes the energy picture is the humble gamma ray.
It's like when you're baking cookies, and the recipe calls for flour, sugar, and eggs. You put them all in, mix them up, and bake them. But then you notice the cookies are a little… flatter than you expected. Maybe you forgot to mention the baking powder in your mental list of ingredients, or maybe the oven temperature was slightly off. The "missing item" can be something subtle that affects the whole outcome. In alpha decay, that subtle thing is often energy, carried away by gamma rays.
And that, my friends, is the fascinating, and sometimes slightly perplexing, world of nuclear decay. Keeps you on your toes, doesn't it? Now, if you'll excuse me, I think I need a simpler analogy for my niece. Maybe atoms are more like bouncy balls that sometimes break into smaller bouncy balls?
