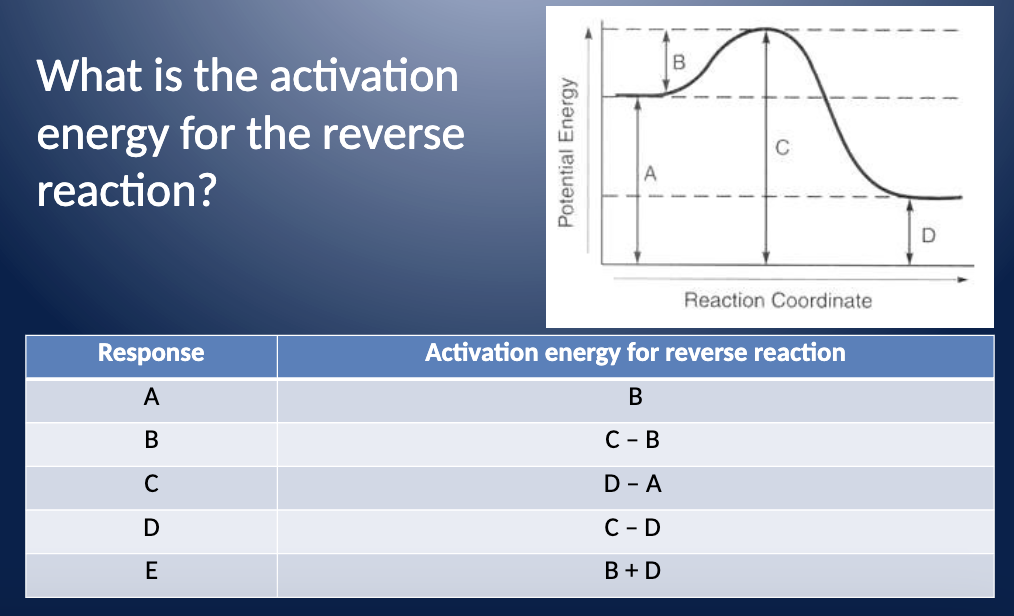
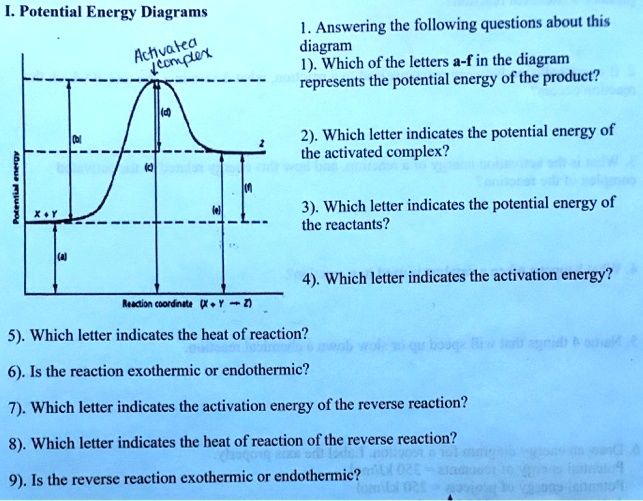
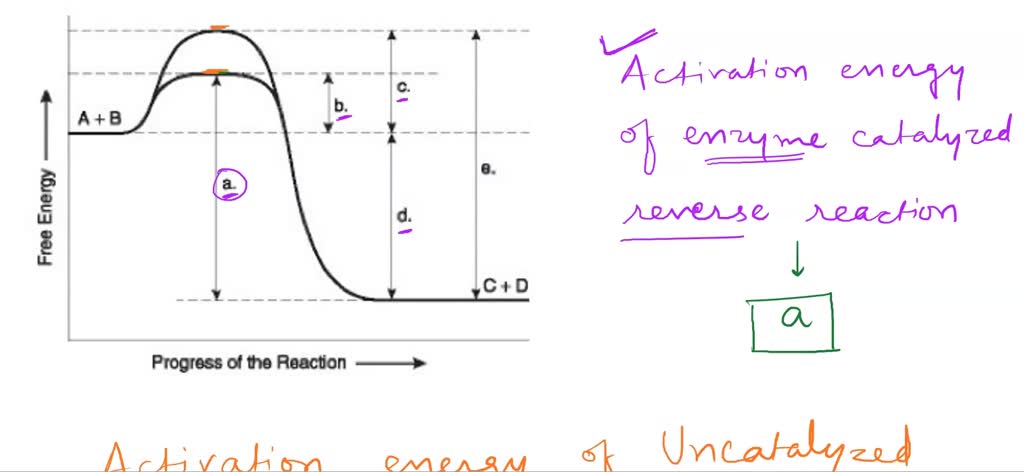
Which Letter Indicates The Activation Energy Of The Reverse Reaction

You know, I was staring at my coffee mug the other day, idly swirling the lukewarm remnants, and a thought, as random as a rogue sock in the dryer, popped into my head. It was about how some things just… happen. You leave a perfectly good slice of cake out, and poof, it's gone. Or, more chemically speaking, you mix two things together, and bam, a reaction occurs. But then, you can sometimes reverse that reaction, right? Like, how does that even work? It got me thinking about the energy involved in these transformations. Not just the energy to start something, but the energy to undo it. And that, my friends, is where we get to the nitty-gritty of activation energy.
Okay, so let's rewind a bit. Remember those chemistry classes? Or maybe you just watched a cool science documentary? You might recall this idea of an "activation energy." It’s basically the minimum amount of energy a reaction needs to get going. Think of it like pushing a boulder uphill. You can't just nudge it a tiny bit and expect it to roll down the other side. You need a good, solid push to get it over that initial hump. That push? That's your activation energy.
So, we've got this activation energy for the forward reaction. That's the one that takes your reactants and turns them into products. Easy enough. But what about going the other way? What if you want to take those products and turn them back into the original reactants? Does it still need a push? And if so, how do we talk about that push? This is where things get a little more interesting, and a tad confusing if you're not paying attention.
The Reverse Reaction's Own Little Hurdle
Think about it logically. If a reaction can go in reverse (and not all reactions can, but let's roll with it for now), then it must also have its own energy barrier to overcome. It's not just a matter of gravity pulling the boulder back down. Sometimes, even to reverse a reaction, you need to give it a little boost. You might need to break some bonds, rearrange some atoms, or overcome some other energetic hurdle. It's like trying to un-bake a cake. You can't just magically get your flour, eggs, and sugar back by wishing it. There's a whole lot of chemical magic (and energy input!) that went into baking it, and getting it back is… well, it’s complicated and energy-intensive!
So, the key question is, what do we call this energy barrier for the reverse reaction? This is where those handy little symbols and letters chemists love come into play. And honestly, sometimes I feel like they invent these things just to keep us on our toes. Or maybe it's just efficient shorthand. Either way, let's break it down.
Decoding the Letters: A Chemist's Secret Handshake
When we talk about activation energy, we usually use the letter E. But that's a bit too broad, right? We need to be more specific. So, we often see a subscript. For the forward reaction, the activation energy is typically denoted as Ea. The 'a' here stands for 'activation'. Simple, right? You’re activating the reactants to become products.
Now, what about the reverse reaction? This is where the intrigue really begins. If Ea is for the forward reaction, and we're talking about the reverse, what letter makes sense? Well, chemists are pretty consistent with their logic, even if it seems a little dry sometimes. If Ea is for the forward activation energy, and we're looking at the reverse path, we need a way to distinguish it. So, what’s the logical opposite of "forward"? It's "reverse," of course! But we don't usually slap a whole word in there. We need a letter.
And that, my dear reader, is where the letter E comes back into play, but with a different modifier to indicate it's the activation energy for the reverse process. While there isn't one single universally mandated letter other than 'E' for activation energy itself, the context and notation become crucial. However, if we're talking about the reverse activation energy, the most common and understood way to represent it, building on the Ea convention, is by using a prime symbol (E'a) or by explicitly stating it in relation to the reverse reaction.
But wait, you might be thinking, "Where's the new letter?" Ah, that's the trick! Often, it's not a new letter, but a modification of the existing one. Think of it like a sequel to a movie. It's still the same franchise, but it's a different installment. Ea is the first installment, and E'a is the follow-up, specifically addressing the reverse path.
The Importance of Context (and a Little Bit of Jargon)
In many textbooks and scientific discussions, you'll see the activation energy of the forward reaction as Ea. When they want to talk about the activation energy of the reverse reaction, they might simply refer to it as the activation energy for the reverse reaction, and if a symbol is used, it's often E'a. The prime symbol (') is a common mathematical and scientific way to denote a related but distinct quantity. So, it's the same concept (activation energy), but applied to the reverse process.
Sometimes, especially in more advanced contexts or specific discussions about kinetics, you might see other notations. For instance, if the forward activation energy is Ea(forward), the reverse might be Ea(reverse). But if we're sticking to single letters and common conventions, the prime is your best bet for a distinct symbol.

Let's get a little more technical, just for a moment, because it helps solidify this. The relationship between the forward and reverse activation energies and the enthalpy change of the reaction (ΔH) is actually quite neat. For an exothermic reaction (one that releases energy, like burning wood), the forward activation energy (Ea) will be higher than the reverse activation energy (E'a). This makes sense, right? It takes more energy to get going than to come back. The reverse reaction is essentially facilitated by the energy released in the forward reaction.
For an endothermic reaction (one that absorbs energy, like photosynthesis), the opposite is true. The reverse activation energy (E'a) will be higher than the forward activation energy (Ea).
The equation that links them is:
ΔH = Ea - E'a
So, you can see that Ea and E'a are intrinsically linked. They are not independent values but are part of the energetic landscape of a reversible reaction. It's like two sides of the same coin, or two different paths up and down a mountain.
Why Does This Even Matter? (Besides Acing Your Next Chemistry Quiz)
Understanding the activation energy of the reverse reaction isn't just an academic exercise. It's crucial for understanding how chemical processes work in the real world. Think about industrial catalysis. Catalysts speed up reactions by lowering the activation energy. But they don't just lower it for the forward reaction; they lower it for both the forward and reverse reactions to achieve equilibrium faster.
Or consider biological systems. Enzymes in your body are fantastic catalysts. They facilitate countless biochemical reactions, and their efficiency is directly related to how they interact with the activation energy barriers of both the forward and reverse reactions. Without understanding these energy landscapes, we wouldn't be able to design new drugs, develop new materials, or even understand how our own bodies function.
It's a bit like trying to build a bridge. You need to know not just how much energy it takes to lay the first stone (forward activation energy) but also how much energy is involved in potentially taking it apart or reinforcing it (reverse activation energy) if things go wrong or need to be modified. It's all about managing those energetic hurdles.
A Little Irony for Your Amusement
Isn't it a bit ironic that the very thing that makes a reaction go – the activation energy – also represents a barrier? It’s like needing a permit to start a business, but that same permit process is also a hurdle you have to overcome. The energy you expend to get going is also the energy you need to stop or undo it in some way. Nature, it seems, loves its little paradoxes.
And the fact that we use a subtle notation like a prime symbol (') to distinguish between forward and reverse activation energies? It’s a testament to the elegance and sometimes infuriating brevity of scientific language. It's like a secret handshake among chemists. If you see E'a, you know they're talking about the reverse activation energy. No need for a whole paragraph; just a little tick mark does the job. Pretty neat, huh? Though, I'll admit, sometimes I miss the days when things were just spelled out in plain English. But then again, imagine how long those textbooks would be!
So, to recap, while the letter E is the fundamental symbol for activation energy, it's the accompanying subscripts and symbols that tell the full story. For the forward reaction, it's typically Ea. For the reverse reaction, the most common symbolic representation is E'a, where the prime symbol distinguishes it from its forward counterpart. It’s not a brand new letter, but a clever modification that carries a world of meaning for those in the know. And now, you’re one of them!
So next time you’re sipping your coffee or watching something chemically interesting happen, you can impress yourself (and maybe a few friends, if you’re feeling bold) with your knowledge of activation energies and their reverse counterparts. It’s just another little piece of the amazing puzzle that is chemistry. And who knows, maybe it’ll inspire another random thought about the energy it takes to simply be.
