Which Is True About The Dissolving Process In Water Brainly

Have you ever watched something just... disappear in water? It's like a tiny magic show happening right before your eyes! Think about when you add sugar to your tea. Poof! It's gone, making your drink perfectly sweet. Or what about that fizzy tablet you drop in water when you're feeling under the weather? It fizzes and bubbles, and then, well, it's not a tablet anymore, is it? These everyday moments are all part of the amazing world of dissolving. And if you're curious about what makes this happen, especially when water is involved, there's a super cool place to find out more: Brainly!
Now, you might be thinking, "Dissolving? That sounds a bit... science-y." But trust me, it's way more fun and interesting than it sounds. Imagine water as a super friendly host at a party. It loves to invite all sorts of things to join in. When something dissolves in water, it's like those invited guests are having such a good time that they break down into super tiny pieces and mingle with all the water molecules. They don't just sit in a lump; they spread out evenly, becoming one with the crowd.
So, what kinds of guests does water like to invite? Well, things like salt and sugar are definitely on the guest list. They're like the life of the party! When you sprinkle salt into a glass of water, those little salt crystals don't stay crystals for long. The water molecules are so energetic and grab onto the individual salt particles, pulling them apart. Soon, the salt is everywhere, spread out so thinly you can't even see it anymore. It's still there, though, just in a much more spread-out, invisible form.
The same thing happens with sugar. That satisfying sweetness you taste? That's the sugar molecules, all spread out and happy amongst the water molecules. It's not like the sugar is gone forever; it's just decided to become part of the water. This process is called dissolution, and water is a fantastic solvent for so many things.
But not everything is invited to water's party. Some things, like sand or tiny pebbles, just aren't made for mingling with water. They're a bit too clumpy, or the water molecules just can't break them down. So, when you put sand in water, it just sits at the bottom. It's like those guests who prefer to stand by the wall and not join the dancing. They're still present, but they haven't dissolved.

This is where things get really interesting and why asking questions is so important. You might wonder, "Why does salt dissolve but not oil?" or "What's the difference between dissolving and just getting wet?" These are exactly the kinds of questions that people on Brainly are asking and answering all the time! It's like a giant, friendly study group for all sorts of curiosities.
Think about it: you can see a fizzy tablet break apart, but it’s different from how a grain of sugar disappears. One involves a chemical reaction creating bubbles, while the other is a purely physical process of molecules separating. Understanding these subtle differences is part of the fun. On Brainly, you can find explanations that break down these concepts in a way that makes sense, even if you're not a science whiz.
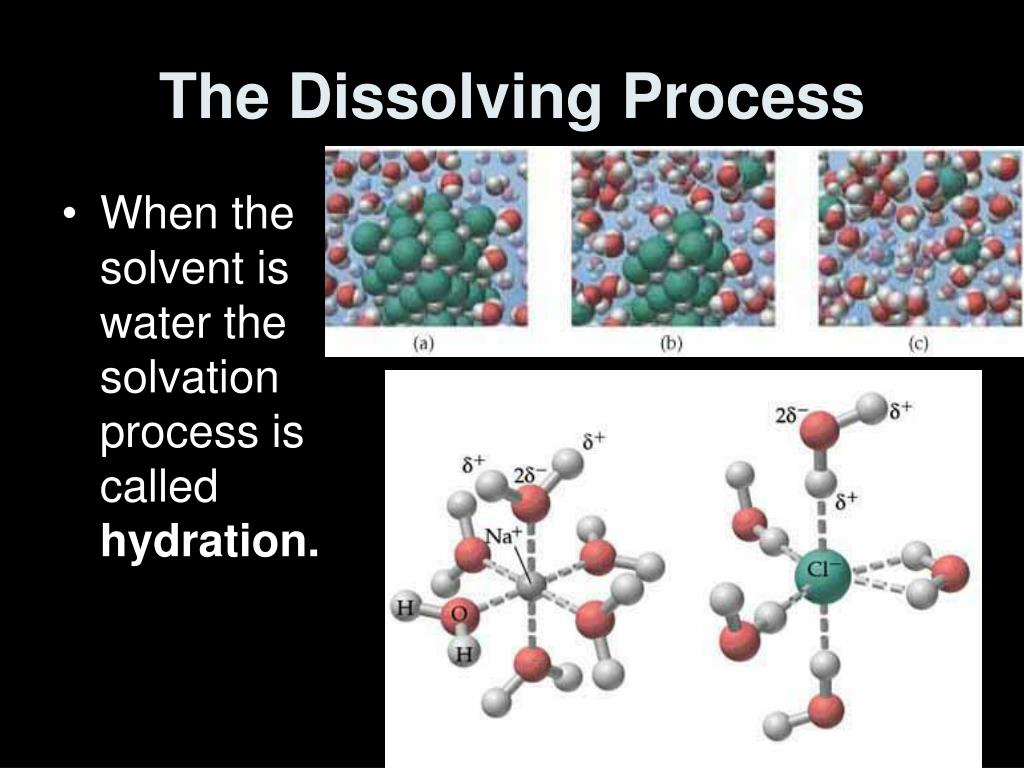
It's like having a friend who's really good at explaining tricky stuff. They can tell you why water is so good at dissolving certain things and not others. It all has to do with how the tiny particles, the molecules, of different substances are shaped and how they interact with each other. Water molecules are like little magnets, and they're really good at attracting and pulling apart the charged parts of other molecules, like those in salt and sugar.
And the best part? You don't have to be a scientist to understand it. Brainly is designed for everyone. You can ask your own questions, and real people, just like you, will offer answers. Or, you can browse through questions that others have already asked. You might find that someone else was wondering the exact same thing you were! It's like a treasure hunt for knowledge.

Have you ever mixed different things in water and seen what happens? Maybe you've tried dissolving glitter (which, spoiler alert, doesn't actually dissolve like sugar!) or seen how food coloring spreads. Each little experiment is a clue. And if you're ever stuck wondering why it happened the way it did, Brainly is your go-to place. They can help you understand the difference between things that dissolve and things that simply disperse or react.
It’s this sense of shared discovery that makes Brainly so special. It's not just about getting the answer; it's about understanding the 'why' behind it all. The dissolving process in water is a perfect example of how simple everyday observations can lead to fascinating scientific insights. So, the next time you see something disappear in your drink or stir something into water, take a moment to appreciate the tiny, invisible party happening. And if you want to dive deeper into the delightful world of dissolving, you know where to look!
