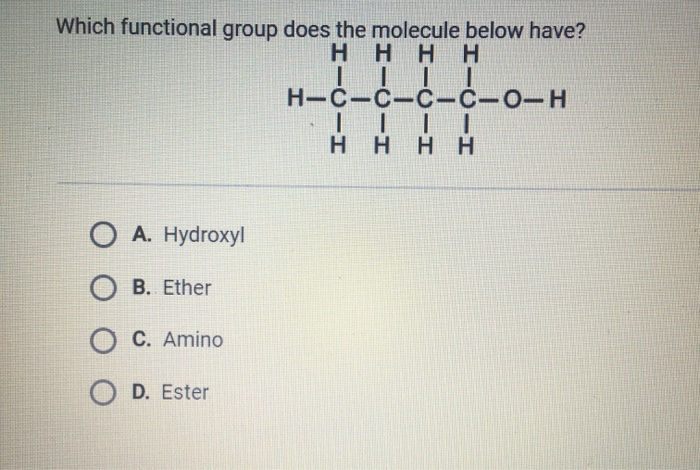
Which Functional Group Does The Molecule Below Have

Ever looked at a molecule and thought, "Wow, that looks… interesting!" Well, get ready for a little chemical adventure. We're about to peek at a molecule that's got a fantastic secret ingredient. It’s like finding a surprise in your favorite snack!
Let's dive in and see what makes this particular arrangement of atoms so much fun. Think of it like a tiny Lego set, but instead of plastic bricks, we have protons, neutrons, and electrons. And when they snap together in just the right way, magic happens!
So, what's the big deal? It all comes down to a special little part of the molecule. This part is called a functional group. It's like the molecule's signature move, its party trick.
Imagine a molecule is a person. The functional group is their personality trait, or maybe their favorite accessory. Is it a sparkly hat? A super-speedy engine? A charming smile? Each functional group gives the molecule its unique character and determines what it can do.
Now, let's get to the star of our show! We're going to look at a molecule that's got a real crowd-pleaser. It’s not just any functional group; it’s one that’s a superstar in the world of chemistry.
Take a good look at the diagram. See that bit right there? The one that looks like a little loop with some extra bits attached? That's our main attraction! It’s the key to unlocking the molecule’s talents.
This specific functional group has a name that might sound a little fancy at first. But don't worry, it's actually super approachable. Drumroll please... it's an alcohol!
Yep, you heard that right – alcohol! Just like the stuff you might find in a refreshing drink (though, please, don't drink any random molecules!). This functional group is a simple but powerful combination of an oxygen atom and a hydrogen atom. They’re bonded together, and that pair is then attached to the rest of the molecule.

So, what makes an alcohol group so special? Well, it’s like giving the molecule a little bit of a “sticky” personality. The oxygen atom in the alcohol group loves to form connections with other molecules. It’s a bit of a social butterfly.
This “stickiness” is what chemists call hydrogen bonding. It’s a super important concept in chemistry. It’s how water molecules hold hands, for example! And it’s a big reason why alcohols behave the way they do.
Think about it: because of this alcohol group, our molecule can dissolve in water much better than if it didn't have it. It’s like it’s wearing a swimsuit and ready to jump into the pool! This makes it useful for all sorts of things.
The alcohol group also influences how the molecule reacts with other chemicals. It can be a starting point for many exciting chemical transformations. It’s like a little doorway to creating even more interesting compounds!
And here’s where it gets really entertaining: many of the things we use and love in our daily lives have this very same alcohol group! It’s a common feature in so many important molecules.
For instance, think about the sugars that give us energy. Many of them are packed with alcohol groups! This is why they can dissolve in our blood and be transported around our bodies. Pretty neat, right?
![Functional Groups in Organic Chemistry [Infographic] | Chemistry.Com.Pk](https://i2.wp.com/chemistry.com.pk/wp-content/uploads/2014/08/Functional-Groups-in-Organic-Chemistry.png)
Even things like the cleaning products under your sink might contain molecules with alcohol groups. They help dissolve grease and grime. So, this little functional group is a real workhorse!
Let’s zoom in even closer on our specific molecule. See that -OH? That’s the shorthand for the alcohol group. The 'O' is for oxygen, and the 'H' is for hydrogen. They’re best buddies, always found together in this setup.
The fun doesn't stop there. Depending on where this -OH group is placed on the molecule, and what else is attached, it can lead to a whole spectrum of different properties and uses. It’s like a chameleon, adapting its behavior!
Some alcohols are small and volatile, like the ones used in hand sanitizers. Others are larger and more complex, found in things like fats and oils. The humble alcohol group is incredibly versatile.
So, when you see that -OH hanging off a molecule, you know you’re looking at something with a bit of flair. It’s a sign of potential, of reactivity, and of connection. It's a tiny powerhouse of chemical possibility.

This is why looking at molecules can be so captivating. Each functional group is like a clue in a fascinating puzzle. It tells us a story about the molecule’s identity and its role in the grand tapestry of chemistry.
It’s the difference between just seeing a jumble of atoms and understanding that they’re arranged in a specific, functional way. It’s like the difference between looking at a pile of bricks and seeing a beautiful house.
The alcohol group is one of the most fundamental and widely recognized functional groups. It’s often one of the first ones students learn about when they start exploring organic chemistry. It’s like the ABCs of molecular building blocks.
And the beauty of it is that it's so common. You encounter molecules with alcohol groups all the time, even if you don't realize it. They’re in the food we eat, the air we breathe, and the materials we use.
So next time you see a molecule diagram, try to spot that little -OH. It’s your ticket to understanding a little bit more about its personality. It’s a signal that this molecule is ready to mingle and make things happen!
It’s a reminder that even the simplest arrangements can lead to complex and wonderful outcomes. The universe is full of these tiny, elegant designs. And learning to recognize them is a journey of discovery.
Think of the joy of recognizing a friend in a crowd. That’s the feeling you get when you spot an alcohol group on a molecule. You know exactly what you’re dealing with, and you can start to predict its behavior.
This simple group is responsible for making many substances water-soluble, which is crucial for life as we know it. Without it, our bodies wouldn’t be able to transport nutrients, and many chemical reactions wouldn’t occur. It’s a true MVP!
It's these fundamental building blocks that make the intricate world of chemistry so accessible and, frankly, so cool. The alcohol group is a perfect example of how a small, defined structure can have a huge impact.
So, while the molecule might look a bit abstract at first glance, knowing about its alcohol functional group gives it a whole new dimension. It’s no longer just a diagram; it's a player in the chemical world, ready to interact and transform.
It’s a little piece of chemical magic, readily identifiable and incredibly important. The alcohol group is a friendly face in the vast universe of molecules. It’s a great place to start your exploration.
Keep an eye out for that -OH. It's a sign of something interesting brewing! And who knows, the next molecule you look at might just surprise you with what it can do. Happy molecule hunting!
