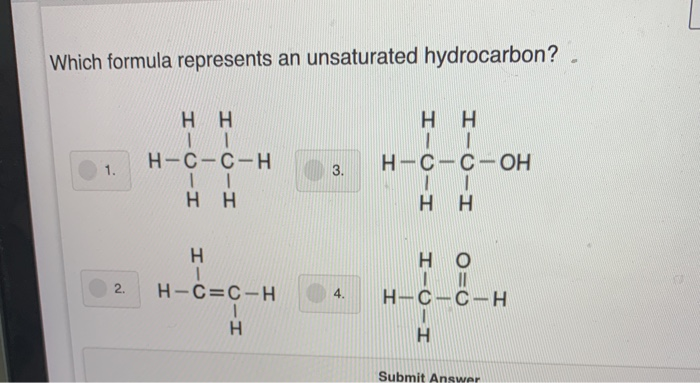
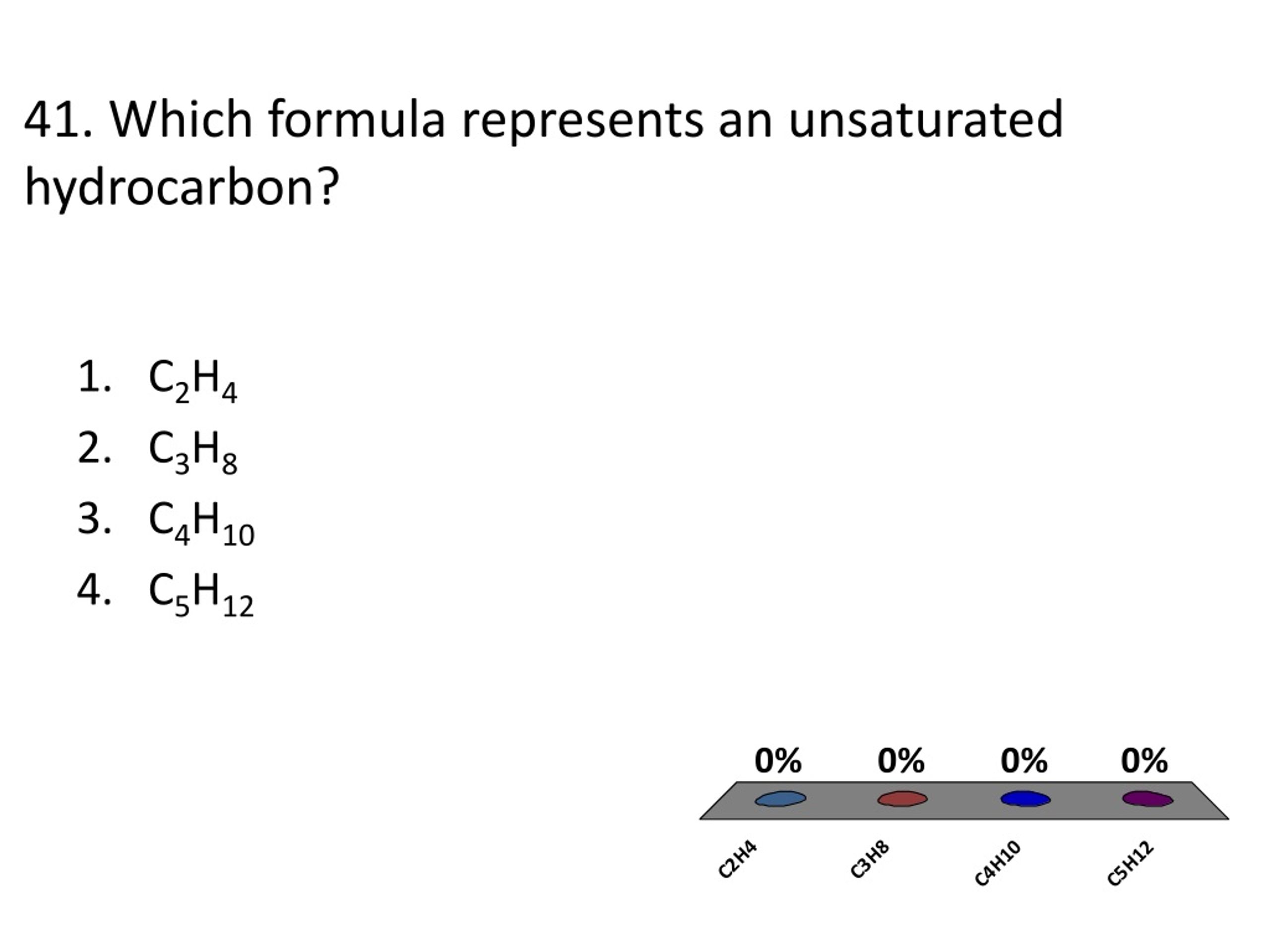
Which Formula Represents An Unsaturated Hydrocarbon

Hey there, chemistry explorers! Ever wonder about the building blocks of, well, everything? Today, we're diving into a super cool part of the chemistry world. We're talking about molecules that have a little extra something special.
Think of it like this: some molecules are pretty straightforward, like a simple string of beads. Others, though, have a bit more pizzazz, with some beads holding hands in a special way. These are our unsaturated hydrocarbons, and they're quite the characters!
So, what makes a hydrocarbon "unsaturated"? It's all about the bonds between the carbon atoms. Carbon atoms love to bond with each other, and they can do it in a few ways.
The most basic way is a single bond, like two friends just holding hands. This is what you find in saturated hydrocarbons. They're perfectly content and stable, like a well-behaved guest.
But in unsaturated hydrocarbons, things get a little more exciting. Here, carbon atoms can form double bonds or even triple bonds. Imagine those friends not just holding hands, but giving each other a double high-five or a super-duper hug!
These double and triple bonds are the secret sauce. They make the molecule more reactive, meaning it's ready for some action. It's like having a toy that's begging to be played with!
Now, let's talk formulas. When chemists describe molecules, they use special shorthand called formulas. These are like secret codes that tell you exactly what's inside.
For saturated hydrocarbons, the general formula is super simple and predictable. It's like a well-worn path. For alkanes, which are the simplest saturated hydrocarbons, the formula is CnH2n+2.
![[Class 10] What are saturated & unsaturated hydrocarbon with examples?](https://d1avenlh0i1xmr.cloudfront.net/large/2b5eeda4-42ba-4d21-a35e-adf9df9e20fb/unsaturated-hydrocarbon-(propane)---teachoo.jpg)
Here, 'n' just represents the number of carbon atoms. So, if you have 1 carbon atom (n=1), you get CH4 (methane). If you have 2 carbon atoms (n=2), you get C2H6 (ethane). It's a neat, tidy pattern.
But when we get to our unsaturated hydrocarbons, the formulas change a bit to reflect those extra bonds. These formulas are where the real fun begins, because they tell a different story.
Let's start with hydrocarbons that have double bonds. These are called alkenes. Because they have a double bond, they have fewer hydrogen atoms compared to their saturated cousins.
The general formula for alkenes with one double bond is CnH2n. See the difference? The '+2' is gone, and that's a big deal in the molecular world!
This means for every two carbon atoms, you'll have only two hydrogens attached to them in that double-bonded section, instead of the usual three. It's like they're sharing their hydrogen friends to form that stronger bond.
For example, ethene (two carbons, one double bond) is C2H4. Compare that to ethane (two carbons, single bond) which is C2H6. That's two fewer hydrogens! This missing hydrogen is what makes it "unsaturated."
Now, let's crank up the excitement to triple bonds. These are called alkynes. Triple bonds are even more intense, meaning even fewer hydrogen atoms can be attached.
The general formula for alkynes with one triple bond is CnH2n-2. Whoa, the formula is going down! The '-2' tells us there are even fewer hydrogens than in alkenes.
So, for two carbon atoms forming a triple bond, we have ethyne (acetylene), which is C2H2. That's a whole lot less hydrogen than ethane (C2H6) or even ethene (C2H4). It's like those carbon atoms are really, really close!
These formulas, CnH2n for alkenes and CnH2n-2 for alkynes, are the key indicators of unsaturation. They are the tell-tale signs that a hydrocarbon molecule has a double or triple bond.
Why is this so entertaining? Because chemistry isn't just about memorizing formulas; it's about understanding the story they tell! These formulas are like little clues in a molecular mystery.

When you see CnH2n or CnH2n-2, your brain should immediately go, "Aha! This molecule is ready to do something interesting!" It's like seeing a lightning bolt symbol – you know there's energy involved.
These unsaturated hydrocarbons are the VIPs in many chemical reactions. They're the ones that readily add other atoms or molecules across their double or triple bonds. Think of it as them being very welcoming hosts, always ready to bring in new guests!
This makes them super important in making all sorts of everyday things. Plastics, for example, often start with these reactive building blocks. So, that cool gadget you're holding? It might have a history with some unsaturated hydrocarbons!
It's like having a Lego brick that can connect in multiple ways, making for more complex and exciting structures. The ability to form double and triple bonds gives these molecules a unique personality.
So, if you're ever looking at a chemical formula and you're not sure if it's saturated or unsaturated, just do a quick check. Does it fit the pattern of CnH2n or CnH2n-2 (for molecules with one double or triple bond, respectively)? If it does, then congratulations, you've found yourself an unsaturated hydrocarbon!
It's a small piece of the puzzle, but it opens up a whole world of chemical possibilities. These molecules are the engines of many transformations in nature and industry.
Think about the smell of a fresh-cut lawn. That scent often comes from volatile organic compounds, many of which are unsaturated hydrocarbons. They are contributing to the sensory experience of our world.
The beauty of chemistry is in these simple yet profound relationships. The formulas are not just abstract symbols; they are blueprints for how molecules behave and interact.
And the unsaturated hydrocarbons are the ones that bring the dynamic action. They're not just hanging around; they're actively participating in the grand dance of chemical reactions.
So, next time you hear about hydrocarbons, remember the distinction. The single-bonded ones are steady and stable, like a reliable friend. But the double and triple-bonded ones? They're the life of the party, ready for adventure!
Keep an eye out for those formulas CnH2n and CnH2n-2. They are your tickets to understanding the exciting world of unsaturated hydrocarbons. It’s a fascinating journey, and these formulas are your first step!
